Emerging Research on Potential Health Effects of Electronic Cigarettes Secondhand Exposure Flavorings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electronic (E-) Cigarettes and Secondhand Aerosol
Defending your right to breathe smokefree air since 1976 Electronic (e-) Cigarettes and Secondhand Aerosol “If you are around somebody who is using e-cigarettes, you are breathing an aerosol of exhaled nicotine, ultra-fine particles, volatile organic compounds, and other toxins,” Dr. Stanton Glantz, Director for the Center for Tobacco Control Research and Education at the University of California, San Francisco. Current Legislative Landscape As of January 2, 2014, 108 municipalities and three states include e-cigarettes as products that are prohibited from use in smokefree environments. Constituents of Secondhand Aerosol E-cigarettes do not just emit “harmless water vapor.” Secondhand e-cigarette aerosol (incorrectly called vapor by the industry) contains nicotine, ultrafine particles and low levels of toxins that are known to cause cancer. E-cigarette aerosol is made up of a high concentration of ultrafine particles, and the particle concentration is higher than in conventional tobacco cigarette smoke.1 Exposure to fine and ultrafine particles may exacerbate respiratory ailments like asthma, and constrict arteries which could trigger a heart attack.2 At least 10 chemicals identified in e-cigarette aerosol are on California’s Proposition 65 list of carcinogens and reproductive toxins, also known as the Safe Drinking Water and Toxic Enforcement Act of 1986. The compounds that have already been identified in mainstream (MS) or secondhand (SS) e-cigarette aerosol include: Acetaldehyde (MS), Benzene (SS), Cadmium (MS), Formaldehyde (MS,SS), Isoprene (SS), Lead (MS), Nickel (MS), Nicotine (MS, SS), N-Nitrosonornicotine (MS, SS), Toluene (MS, SS).3,4 E-cigarettes contain and emit propylene glycol, a chemical that is used as a base in e- cigarette solution and is one of the primary components in the aerosol emitted by e-cigarettes. -
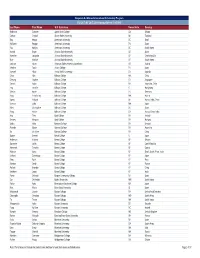
Gilman Fall 2017 Recipients for Website (1.4.18).Xlsx
Benjamin A. Gilman International Scholarship Program Fall 2017/AY 2017-2018 Awards Offered (1/4/2018) Last Name First Name U.S. Institution Home State Country Robinson Summer Agnes Scott College GA Ghana Cotton Crystal Alcorn State University MI Thailand Roy Kenya American University DC Brazil Williams Reagan American University TN Spain You Kaitlyn American University DC South Korea Arriola Bryan Arizona State University AZ Spain Kaercher Jacquelin Arizona State University AZ Czech Republic Ruiz Maribel Arizona State University AZ South Korea Jackson Julian Arkansas State University, Jonesboro AR Austria Medina Paola Austin College TX Spain Samuel Alicia Azusa Pacific University CA Uganda Chau Alex Babson College MA China Cheung Daphne Babson College CA Singapore Dennis Aidan Babson College NY Argentina, Chile Eng Jennifer Babson College CT Hong Kong Macias Karen Babson College TX Germany Ning Priscilla Joy Babson College MA Austria Spears Mikayla Babson College WI Russia, India, China Stetson Lydia Babson College MA Japan West Christopher Babson College LA Spain Yang Austin Babson College CA Russia, China, India Hoy Theo Bard College NY Iceland Kenney Meagan Bard College NY Hungary Gadio Haby Barnard College NY Senegal Paredes Elanie Barnard College NY Argentina Xu Christine Barnard College NY China Baxter Everett Beloit College IL Japan Anderson Kristina Berea College KY Bhutan Bannister Caleb Berea College KY Czech Republic Hammett Timothy Berea College KY Cyprus Hobson Kyree Berea College KY Brazil, South Africa, India Jenkins Dominique -

BRFSS Brief Electronic Cigarette
BRFSS Brief Number 2020-01 The Behavioral Risk Factor Surveillance System (BRFSS) is an annual statewide telephone survey of adults developed by the Centers for Disease Control and Prevention (CDC) and administered by the New York State Department of Health. The BRFSS is designed to provide information on behaviors, risk factors, and utilization of preventive services related to the leading causes of chronic and infectious diseases, disability, injury, and death among the noninstitutionalized, civilian population aged 18 years and older. Electronic Cigarette Use New York State Adults, 2017 Introduction and Key Findings Electronic cigarettes (e-cigarettes) are battery-powered devices that heat a solution of liquid nicotine, flavorings, and other chemicals creating an aerosol that is inhaled by the user. E-cigarettes are known by many different names including e-cigs, vapes, vape pens, e-hookahs, and electronic nicotine delivery systems (ENDS). Using an e-cigarette is called vaping. E-cigarettes are not a United States (US) Food and Drug Administration (FDA) approved smoking cessation aid and their usefulness as a cessation aid is unproven. With or without nicotine, e-cigarettes are not hazard-free and e- cigarette aerosol is not simply water vapor; the aerosol may contain heavy metals, volatile organic compounds, ultrafine particles, and other toxins.1 In addition, e-cigarette use can undermine social norms about tobacco, delay cessation among cigarette smokers, and increase the risk of ever using combustible tobacco cigarettes among youth and young adults.1 The long-term health risks of e-cigarettes will not be known for decades. The FDA has extended regulatory authority to all tobacco products including e-cigarettes.2 But the FDA approach to regulation of e-cigarettes is being phased in over time, may be delayed by litigation, and effective regulation may be years away. -

Banning Flavored E-Cigarettes
CONTENTS Executive Summary 1 Introduction 2 The Potential for Unintended Harms 3 Predicting Response to a Flavor Ban 3 Impacts of a Flavor Ban on Harm Reduction 5 Other Impacts of a Flavor Ban 7 Lost Revenue and the Potential Public Health Tradeoff 8 Conclusion 8 About the Author 9 sation efforts and the growth of counterfeit and contraband products; and harm to communities via lost funding for broader health resources. A limited number of studies have looked at people’s actual and presumptive responses to flavor bans. This small body of research suggests that the policy could reduce vaping in R STREET POLICY STUDY NO. 222 general, but that it may drive some current vapers to resume March 2021 or increase their use of combustible cigarettes and others to seek out their preferred e-cigarette flavors through illicit markets and hard-to-regulate online retailers. As such, both potential sets of behavior changes could tip the net public health impact of flavor bans toward harmful. BANNING FLAVORED Because ENDS users inhale a nicotine-infused vapor rath- E-CIGARETTES COULD HAVE er than toxin-laden tobacco smoke, vaping is considered a safer alternative to smoking combustible cigarettes. In fact, UNINTENDED PUBLIC HEALTH both the U.S. Centers for Disease and Prevention and Public CONSEQUENCES Health England have stated (albeit to varying degrees) that smokers would benefit from switching to e-cigarettes, and By Stacey A. McKenna the devices are gaining traction as cessation tools. Further- more, research shows that flavors may aid individuals who are using e-cigarettes to quit or reduce smoking. -

Juul and Other High Nicotine E-Cigarettes Are Addicting a New Generation of Youth
JUUL AND OTHER HIGH NICOTINE E-CIGARETTES ARE ADDICTING A NEW GENERATION OF YOUTH Launched in 2015, JUUL quickly disrupted the e-cigarette marketplace, popularizing e-cigarette devices that are sleek, discreet and have sweet flavors and a powerful nicotine hit. Nicotine is highly addictive, can negatively impact the development of the adolescent brain, and can harm the cardiovascular system.1 Youth e-cigarette use in the United States has skyrocketed to what the U.S. Surgeon General and the FDA have called “epidemic” levels, with 3.6 million middle and high school students using e- cigarettes. 2 Former FDA Commissioner Scott Gottlieb has stated, “There’s no question the Juul product drove a lot of the youth use.”3 The Surgeon General has called for “aggressive steps to protect our children from these highly potent products that risk exposing a new generation of young people to nicotine.”4 Use of Nicotine Salts Makes it Easier for New Users to Try E-Cigarettes Just like the tobacco industry has used additives and design changes to make cigarettes more addictive and appealing to new users (particularly youth),5 JUUL pioneered a new e-liquid formulation that delivers nicotine more effectively and with less irritation than earlier e-cigarette models. According to the company, the nicotine in JUUL is made from “nicotine salts found in leaf tobacco, rather than free-base nicotine,” in order to “accommodate cigarette-like strength nicotine levels.”6 JUUL’s original patent stated that, “certain nicotine salt formulations provide satisfaction in an individual superior to that of free base nicotine, and more comparable to the satisfaction in an individual smoking a traditional cigarette. -

Associate Leadership Institute
diversity Associate Leadership Institute 2018 PARTICIPANT DIRECTORY www.nycbar.org/ALI 2018 FELLOWS INDEX Adam Acosta, White & Case LLP Rashida Adams, White & Case LLP Dupe Adegoke, Reed Smith LLP Randa Adra, Crowell & Moring LLP Nelly Almeida, Milbank, Tweed, Hadley & McCloy LLP Sarah Anstey, Fragomen, Del Rey, Bernsen & Loewy, LLP Christopher Avellaneda, Schulte Roth & Zabel LLP Nairuby Beckles, Paul, Weiss, Rifkind, Wharton & Garrison LLP Camille Bent, BakerHostetler LLP Sally Bergmann, Debevoise & Plimpton LLP Tsedey Bogale, Reed Smith LLP Adrienne Bradley, Sullivan & Cromwell LLP Amanda A. Butler-Jones, Akin Gump Strauss Hauer & Feld LLP Marcie Cleary, Frankfurt Kurnit Klein & Selz PC Marissa Comart, Davis & Gilbert LLP Elizabeth Dahill, Seyfarth Shaw LLP Christopher Davis, Kramer Levin Naftalis & Frankel LLP Ekta Dharia, MoloLamken LLP Audra Dowless, Schulte Roth & Zabel LLP Aleesha Fowler, McGuireWoods LLP Luke Frankson, Sidley Austin LLP Rebecca R. Friedman, Kasowitz Benson Torres LLP Sharonmoyee Goswami, Cravath, Swaine & Moore LLP Chas Hamilton, Paul, Weiss, Rifkind, Wharton & Garrison LLP Brian Harris, Ropes & Gray LLP Antonio Haynes, Davis Polk & Wardwell LLP Simone Hicks, Debevoise & Plimpton LLP Susan Hu, Arnold & Porter LLP Keith A. James Jr., Paul, Weiss, Rifkind, Wharton & Garrison LLP Aileen Kim, Weil, Gotshal & Manges LLP Christina Kim, Davis Wright Tremaine LLP 1 | City Bar Associate Leadership Institute | 2018 Julia Kim, Sullivan & Cromwell LLP Joyce Kwok, Sullivan & Cromwell LLP Cassandra Labbees, Epstein Becker and Green PC Justin Lee, White & Case LLP Victoria Lee, WilmerHale Gary Lo, Davis Polk & Wardwell LLP Deborah Kemi Martin, Dechert LLP Katie McShane, Cadwalader Wickersham & Taft LLP Silvia Medina, White & Case LLP Marco Molina, BakerHostetler LLP Nadine F. Mompremier, Ropes & Gray LLP Rohit Nafday, Wachtell, Lipton, Rosen and Katz Marianna Ofosu, Wachtell, Lipton, Rosen and Katz Byron Pacheco, Boies Schiller Flexner LLP Andrew P. -

Monitoring Changing Tobacco Use Behaviors: 2000 - 2014
Monitoring Changing Tobacco Use Behaviors: 2000 - 2014 Maryland Department of Health and Mental Hygiene Cigarette Restitution Fund Center for Tobacco Prevention and Control State Fiscal Year 2015 Larry Hogan Governor State of Maryland Boyd Rutherford Lieutenant Governor State of Maryland Van Mitchell Secretary Department of Health and Mental Hygiene Statutory Authority and Requirements Maryland’s Health-General Article, Title 13, Subtitle 10, requires the Maryland Department of Health and Mental Hygiene (DHMH) to conduct a biennial tobacco study and report specific findings to the Maryland Governor and the General Assembly. The appendices to this report provide the detailed data for indicators DHMH is required to report in its biennial tobacco study for underage youth. THIS PAGE HAS BEEN LEFT BLANK INTENTIONALLY 1 Table of Contents Suggested Citation ......................................................................................................................... 5 Cover Letter ..................................................................................................................................... 6 In Brief ............................................................................................................................................... 8 Commonly Used Acronyms Found in this Report ..................................................................... 11 About this Report .......................................................................................................................... 12 Data in this -

Regulating E-Cigarettes Regulating E-Cigarettes
Regulating E-cigarettes Regulating E-Cigarettes The introduction of electronic cigarettes (or e-cigarettes) in countries around the world poses new challenges to governments wanting to protect youth and reduce tobacco use. Policies governing e-cigarettes must be guided by an assessment of the impact of these products on the pace of progress in reducing the death and disease caused by tobacco use. The evidence around the health harms from e-cigarettes and their impact on young people is evolving. Any assessment of the evidence has to consider the impact on both individual smokers and the population as a whole. While e-cigarettes may have health benefits to individual smokers if they are proven to help smokers quit completely, they are nevertheless harmful to public health if they lead to more young people starting, if they renormalize tobacco use, and/or if they discourage smokers from quitting. The World Health Organization has concluded that e-cigarettes are “undoubtedly harmful” and that countries “that have not banned [e-cigarettes] should consider regulating them as harmful products.” 1 This is consistent with the general obligations of the WHO Framework Convention on Tobacco Control, which require Parties to the Convention to implement measures for preventing and reducing nicotine addiction. 2 In the absence of effective government regulation, e-cigarettes could create a new generation of nicotine and tobacco users and undermine the progress made in combatting the tobacco epidemic. This policy brief uses a three-step process to assist government officials in determining how to regulate e-cigarettes to fit their country circumstances while balancing competing public health considerations: 1. -

Vape Shops' in the San Francisco Bay Area
UCSF UC San Francisco Previously Published Works Title A Pilot Study of Retail 'Vape Shops' in the San Francisco Bay Area. Permalink https://escholarship.org/uc/item/9nv2x6r0 Journal Tobacco prevention & cessation, 2(Suppl) ISSN 2459-3087 Authors Burbank, Andrea D Thrul, Johannes Ling, Pamela M Publication Date 2016 DOI 10.18332/tpc/65229 Peer reviewed eScholarship.org Powered by the California Digital Library University of California HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Tob Prev Manuscript Author Cessat. Author Manuscript Author manuscript; available in PMC 2017 April 06. Published in final edited form as: Tob Prev Cessat. 2016 ; 2(Suppl): . doi:10.18332/tpc/65229. A Pilot Study of Retail ‘Vape Shops’ in the San Francisco Bay Area Andrea D Burbanka,*, Johannes Thrula,*, and Pamela M Linga aUniversity of California, San Francisco, United States Abstract INTRODUCTION—The use of electronic cigarettes or vape devices is increasing, and products are evolving rapidly. This study assessed retail vape shops in the San Francisco Bay Area to describe store characteristics, products offered, advertisements and health claims, as well as employees’ perceptions of their customers’ demographics, and practices to support smoking cessation. METHODS—We conducted store audits of shops that exclusively sell vape devices with physical addresses in San Francisco and Alameda counties (n=23, response rate 72%) and interviewed vape shop owners/employees. RESULTS—While all stores carried second and third generation vape devices, 83% of stores did not carry first generation devices. Employees estimated the majority of their customers bought devices for smoking cessation or to replace tobacco, and a small minority purchased for first-time recreational use. -

Information to Users
INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. UMI University Microfilms International A Bell & Howell Information Company 300 Nortfi Zeeb Road, Ann Arbor, Ml 48106-1346 USA 313/761-4700 800/521-0600 Order Number 9227230 The development and performance of chromium/ reactive element-modified aluminide diffusion coatings by chloride-activated pack cementation Bianco, Robert, Ph.D. -

White Paper on E-Cigarettes
7 Cedar St., Suite A Summit, NJ 07901 Phone: 908-273-9368 Fax: 908-273-9222 Email: [email protected] www.njgasp.org Karen Blumenfeld, Director Tobacco Control Policy and Legal Resource Center [email protected] January 22, 2015 ELECTRONIC SMOKING DEVICES Introduction The electronic smoking device industry has evolved and now offers more products than electronic cigarettes, like hookah pens and electronic cigars. Some industry trade groups refer to these products as Personal Electronic Vaporizing Units (PEVUs). Throughout this paper, the term “e-cigarette” is used broadly to include all types of electronic smoking devices. Global Advisors on Smokefree Policy1 (“GASP”) has many health concerns regarding e-cigarette use and exposure, which are documented in this paper. The U.S. Food & Drug Administration (FDA), U.S. Senator Frank Lautenberg, the World Health Organization, and national advocacy organizations also voice their concerns about e-cigarettes. Local, county, state and international jurisdictions are restricting or banning the sale or use of e- cigarette products. I. Laws and Policies New Jersey restricts e-cigarette sales and use New Jersey was the first state in the nation to ban the use of e-cigarettes in public places and workplaces, effective March 13, 2010. On January 11, 2010, Governor Corzine signed into law A4227/A4228/S3053/S3054, banning e-cigarette use in public places and workplaces (amended 2006 NJ Smokefree Air Act), and banning e-cigarette sales to people 18 years and younger. The New Jersey Senate and Assembly both voted unanimously in favor of the law. See http://njgasp.org/sfaa_2010_w-ecigs.pdf and njleg.state.nj.us/2008/Bills/A4500/4227_U1.pdf. -

(ZTA) 19-06 Would Add Vape Shop As a Use Allowed in Certain Zones As a Limited Use and Establish the Limited Use Standards for a Vape Shop
MONTGOMERY COUNTY PLANNING DEPARTMENT THE MARYLAND-NATIONAL CAPITAL PARK AND PLANNING COMMISSION MCPB Item No. 4 Date: 10-17-19 Zoning Text Amendment (ZTA) No. 19-06, Vape Shops - Limited Use; Bill 29-19/Bill 31-19/Bill 32-19, Health and Sanitation – Electronic Cigarettes – Distribution, Use, and Possession/ Flavored Electronic Cigarettes Gregory Russ, Planner Coordinator, FP&P, [email protected], 301-495-2174 Jason Sartori, Chief, FP&P, [email protected], 301-495-2172 Completed: 10/10/19 Description Zoning Text Amendment (ZTA) 19-06 would add Vape Shop as a use allowed in certain zones as a limited use and establish the limited use standards for a Vape Shop. Three companion bills were also introduced to be adopted as Board of Health Regulations. Bill 29-19 would prohibit an electronic smoking devices manufacturer from distributing electronic cigarettes to retail stores within a certain distance of certain schools. Bill 31-19 would prohibit the distribution of any tobacco product, coupon redeemable for a tobacco product, cigarette rolling paper, or electronic cigarette to any individual under 21 except under certain circumstances. It would also prohibit an individual under 21 from using or possessing a tobacco product or electronic cigarette except under certain circumstances. Bill 32-19 would prohibit an electronic smoking devices manufacturer from distributing flavored electronic cigarettes to certain retail stores. This staff report addresses the changes to the Zoning Ordinance (ZTA provisions) and includes the companion bills for informational and contextual purposes only. Summary Staff recommends approval, with modifications, of ZTA 19-06 to add Vape Shop as a use allowed in certain zones as a limited use and establish the limited use standards for a Vape Shop.