NASCE2011 FINAL Program
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Peripheral Nervous System……………………………………………….9
PhD School in Integrative Biomedical Research Department of Pharmacological and Biomolecular Sciences Curriculum: Neuroscience Molecular basis for the development of innovative therapies for peripheral neuropathies treatment: role and cross-regulation of the GABAergic system and neuroactive steroids SDD BIO/09 - Physiology Luca Franco Castelnovo Badge number R10402 PhD Tutor: Prof. Valerio Magnaghi PhD School Coordinator: Prof. Chiarella Sforza Academic year 2015/2016 INDEX Abstract…………………………………………………………………...page 1 Abbreviations list…………………………………………………………….....5 Introduction………………………………………………………………….....8 Peripheral nervous system……………………………………………….9 General concepts……………...……….……………………………........……..9 Sensory system and nociceptive fibers.………………..………….…………..12 Schwann cells and myelination……….……..………………...………………15 Peripheral neuropathies…….…………………………………………...26 General concepts……………………………………………………...……….26 Neuropathic pain……………………………………...……………………….27 Nerve regeneration…………………………………………………………….28 The GABAergic system………….……………………………………...32 GABA……………….…………………………………………………..……..32 GABA-A receptors…………………………………………………………….33 GABA-B receptors…………………………………………………………….42 GABAergic system in the peripheral nervous system…………………………47 Protein kinase C – type ε……………………….………………………..51 General concepts…………………………………………………….…………51 Cross-talk with allopregnanolone and GABA-A………………………………54 Neuroactive steroids…………......………………………………………57 General concepts…………………………………….…………………………57 Mechanism of action…………………………….……………………………..59 Progesterone derivatives………………………………….……………………60 -

Nicotinic Signalling and Neurosteroid Modulation in Principal Neurons of the Hippocampal Formation and Prefrontal Cortex
Nicotinic Signalling and Neurosteroid Modulation in Principal Neurons of the Hippocampal Formation and Prefrontal Cortex by Beryl Yik Ting Chung A Thesis presented to The University of Guelph In partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biomedical Sciences and Neuroscience Guelph, Ontario, Canada © Beryl Yik Ting Chung, April, 2018 ABSTRACT NICOTINIC SIGNALLING AND NEUROSTEROID MODULATION IN PRINCIPAL NEURONS OF THE HIPPOCAMPAL FORMATION AND PREFRONTAL CORTEX Beryl Yik Ting Chung Advisor: University of Guelph, 2018 Dr. Craig D.C. Bailey Nicotinic signalling plays an important role in coordinating the response of neuronal networks in many brain regions. During pre- and postnatal circuit formation, neurotransmission mediated by nicotinic acetylcholine receptors (nAChRs) influences neuronal survival and regulates neuronal excitability, synaptic transmission, and synaptic plasticity. Nicotinic signalling is also necessary for the proper function of the hippocampal formation (HF) and prefrontal cortex (PFC), which are anatomically and functionally connected and facilitate higher-order cognitive functions. The decline or dysfunction in nicotinic signalling and nAChR function has been observed in various neurological disorders, and the disruption or alteration of nicotinic signalling in the HF and/or PFC can impair learning and memory. While the location and functional role of the α4β2* nAChR isoform has been well characterized in the medial portion of the PFC, this is not well-established in the HF. What is the role of α4β2* nAChRs in excitatory principal neurons of the HF during early development? Growing evidence suggests that the progesterone metabolite allopregnanolone (ALLO) plays a role in mediating the proper function of the HF and the PFC, and that it may also inhibit nAChR function. -

A Comprehensive Multilocus Assessment of Sparrow (Aves: Passerellidae) Relationships ⇑ John Klicka A, , F
Molecular Phylogenetics and Evolution 77 (2014) 177–182 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Short Communication A comprehensive multilocus assessment of sparrow (Aves: Passerellidae) relationships ⇑ John Klicka a, , F. Keith Barker b,c, Kevin J. Burns d, Scott M. Lanyon b, Irby J. Lovette e, Jaime A. Chaves f,g, Robert W. Bryson Jr. a a Department of Biology and Burke Museum of Natural History and Culture, University of Washington, Box 353010, Seattle, WA 98195-3010, USA b Department of Ecology, Evolution, and Behavior, University of Minnesota, 100 Ecology Building, 1987 Upper Buford Circle, St. Paul, MN 55108, USA c Bell Museum of Natural History, University of Minnesota, 100 Ecology Building, 1987 Upper Buford Circle, St. Paul, MN 55108, USA d Department of Biology, San Diego State University, San Diego, CA 92182, USA e Fuller Evolutionary Biology Program, Cornell Lab of Ornithology, Cornell University, 159 Sapsucker Woods Road, Ithaca, NY 14950, USA f Department of Biology, University of Miami, 1301 Memorial Drive, Coral Gables, FL 33146, USA g Universidad San Francisco de Quito, USFQ, Colegio de Ciencias Biológicas y Ambientales, y Extensión Galápagos, Campus Cumbayá, Casilla Postal 17-1200-841, Quito, Ecuador article info abstract Article history: The New World sparrows (Emberizidae) are among the best known of songbird groups and have long- Received 6 November 2013 been recognized as one of the prominent components of the New World nine-primaried oscine assem- Revised 16 April 2014 blage. Despite receiving much attention from taxonomists over the years, and only recently using molec- Accepted 21 April 2014 ular methods, was a ‘‘core’’ sparrow clade established allowing the reconstruction of a phylogenetic Available online 30 April 2014 hypothesis that includes the full sampling of sparrow species diversity. -

A COMPARATIVE ANALYSIS of SONG and RESPONSES to SONG PLAYBACK University of Colorado at Denver
FINAL REPORT A COMPARATIVE ANALYSIS OF SONG AND RESPONSES TO SONG PLAYBACK IN THE AVIAN GENUS PIPILO Peter S. Kaplan Department of Psychology University of Colorado at Denver Abstract An experiment was undertaken to characterize the responses of Green-tailed Towhees (Pipilo chlorura) and Rufous-sided Towhees (P. erythophthalmus) to each others' songs and to the songs of five other towhee species, plus one hybrid form. A total of 12 Green-Tailed Towhees and 10 Rufous-sided Towhees from Boulder and Gilpin counties were studied at three field sites: the Doudy Draw Trail, the National Center for Atmospheric Research (NCAR), and on private land at the mouth of Coal Creek Canyon. In May, each bird was mist-netted and banded to facilitate individual identification. During the subsequent playback phase in June and July, each individual received one three-part playback trial on each of 7 consecutive or near-consecutive days. A 9-min playback trial consisted of a 3-min "pre-play" period, during which the bird was observed in the absence of song playback, a 3-min "play" period, in which tape recorded song was played to the subject from a central point in his territory, and a 3-min "post-play" period when the bird • was again observed in the absence of song playback. Order of presentation of song exemplars from different towhee species were randomized across birds. The main dependent measure was the change in the number of songs produced by the subject bird during song playback, relative to the pre-play period. Results showed that Green-tailed Towhees responded by significantly increasing their rate of singing, but only in response to Green-tailed Towhee songs. -

21 Sep 2018 Lists of Victims and Hosts of the Parasitic
version: 21 Sep 2018 Lists of victims and hosts of the parasitic cowbirds (Molothrus). Peter E. Lowther, Field Museum Brood parasitism is an awkward term to describe an interaction between two species in which, as in predator-prey relationships, one species gains at the expense of the other. Brood parasites "prey" upon parental care. Victimized species usually have reduced breeding success, partly because of the additional cost of caring for alien eggs and young, and partly because of the behavior of brood parasites (both adults and young) which may directly and adversely affect the survival of the victim's own eggs or young. About 1% of all bird species, among 7 families, are brood parasites. The 5 species of brood parasitic “cowbirds” are currently all treated as members of the genus Molothrus. Host selection is an active process. Not all species co-occurring with brood parasites are equally likely to be selected nor are they of equal quality as hosts. Rather, to varying degrees, brood parasites are specialized for certain categories of hosts. Brood parasites may rely on a single host species to rear their young or may distribute their eggs among many species, seemingly without regard to any characteristics of potential hosts. Lists of species are not the best means to describe interactions between a brood parasitic species and its hosts. Such lists do not necessarily reflect the taxonomy used by the brood parasites themselves nor do they accurately reflect the complex interactions within bird communities (see Ortega 1998: 183-184). Host lists do, however, offer some insight into the process of host selection and do emphasize the wide variety of features than can impact on host selection. -

Life History Account for California Towhee
California Wildlife Habitat Relationships System California Department of Fish and Wildlife California Interagency Wildlife Task Group CALIFORNIA TOWHEE Melozone crissalis Family: EMBERIZIDAE Order: PASSERIFORMES Class: AVES B484 Written by: D. Dobkin, S. Granholm Reviewed by: L. Mewaldt Edited by: R. Duke Updated by: CWHR Program Staff, November 2014 DISTRIBUTION, ABUNDANCE, AND SEASONALITY The former brown towhee recently has been split into the California towhee and the canyon towhee, M. fusca (American Ornithologists' Union 1989). The California towhee is a common, characteristic resident of foothills and lowlands in most of cismontane California. Frequents open chaparral and coastal scrub, as well as brush-land patches in open riparian, hardwood hardwood-conifer, cropland, and urban habitats. Commonly uses edges of dense chaparral and brushy edges of densely wooded habitats. Also occurs in lowest montane habitats of similar structure in southern California, and locally in Siskiyou and western Modoc cos. Local on coastal slope north of southern Humboldt Co., and apparently absent from western San Joaquin Valley (Grinnell and Miller 1944, McCaskie et al. 1979, Garrett and Dunn 1981). The Inyo California towhee, M. c. eremophilus, occurs only in the Argus Mountains of southwestern Inyo Co. SPECIFlC HABITAT REQUIREMENTS Feeding: Feeds on seeds, insects, and some fruits. Gleans and scratches in litter, picks seeds and fruits from plants, and rarely flycatches (Davis 1957). Prefers to forage on open ground adjacent to brushy cover. Insects are important in breeding season, often constituting a third of the diet (Martin et al. 1961). Cover: Shrubs in broken chaparral, margins of dense chaparral, willow thickets, and brushy understory of open wooded habitats provide cover. -

Voice in Communication and Relationships Among Brown Towhees
THE CONDOR VOLUME 66 SEPTEMBER-OCTOBER, 1964 NUMBER 5 VOICE IN COMMUNICATION AND RELATIONSHIPS AMONG BROWN TOWHEES By JOET.MARSHALL,JR. This paper seeks to answer two questions: (1) What is the function of each song and call in brown towhees; that is, what information does a bird communicate to its fellows vocally, o’r how does it regulate their behavior by its voice? (2) What evi- dence does voice offer for understanding relationship by descent within the closely-knit group of brown towhee species? For the first, I would extend the analysis of Quain- tance (1938,194l) to all members of the group. As to the second question, an ingenious evolutionary reconstruction, based on museum and habitat studies, has been developed by Davis (1951). Do vocal attributes agree with his scheme? The three speciesof brown towhees, genus Pipdo, are the same size and general color and are more similar to each other than any one of them is to other ground-inhabiting finches in the same genus and in the genus Melozorte. Indeed, so close is their relation- ship that the same calls can easily be discerned in each species; although differing in timbre, similarity in form and usage proclaims them to be homologous. The Abert Towhee (Pipdo abed) occupies dense riparian woodland and mesquite thickets of the Colorado River and Gila River drainages, mostly in Arizona. The Brown Towhee proper (Pip20 fuscus) lives in brushy margins of openings in the southwestern United States and Mexico. The White-throated Towhee (Pipilo aZbicoZZis)inhabits brushy slopes, often with tree yuccas, in Puebla and Oaxaca, MCxico. -

PRODUCT SPECIFICATION Anti-PAQR9 Product
Anti-PAQR9 Product Datasheet Polyclonal Antibody PRODUCT SPECIFICATION Product Name Anti-PAQR9 Product Number HPA052798 Gene Description progestin and adipoQ receptor family member IX Clonality Polyclonal Isotype IgG Host Rabbit Antigen Sequence Recombinant Protein Epitope Signature Tag (PrEST) antigen sequence: IMLESWLFDLRGENPTLFVHF Purification Method Affinity purified using the PrEST antigen as affinity ligand Verified Species Human Reactivity Recommended IHC (Immunohistochemistry) Applications - Antibody dilution: 1:20 - 1:50 - Retrieval method: HIER pH6 Characterization Data Available at atlasantibodies.com/products/HPA052798 Buffer 40% glycerol and PBS (pH 7.2). 0.02% sodium azide is added as preservative. Concentration Lot dependent Storage Store at +4°C for short term storage. Long time storage is recommended at -20°C. Notes Gently mix before use. Optimal concentrations and conditions for each application should be determined by the user. For protocols, additional product information, such as images and references, see atlasantibodies.com. Product of Sweden. For research use only. Not intended for pharmaceutical development, diagnostic, therapeutic or any in vivo use. No products from Atlas Antibodies may be resold, modified for resale or used to manufacture commercial products without prior written approval from Atlas Antibodies AB. Warranty: The products supplied by Atlas Antibodies are warranted to meet stated product specifications and to conform to label descriptions when used and stored properly. Unless otherwise stated, this warranty is limited to one year from date of sales for products used, handled and stored according to Atlas Antibodies AB's instructions. Atlas Antibodies AB's sole liability is limited to replacement of the product or refund of the purchase price. -

Biological Inventory and Evaluation of Conservation Strategies in Southwest Playa Wetlands
Biological Inventory and Evaluation of Conservation Strategies in Southwest Playa Wetlands Final Report to the Nebraska Game and Parks Commission and the Playa Lakes Joint Venture August 2007 Alison Banks Cariveau, Research Division Director Lacrecia Johnson, Playa Survey Project Leader Robert Sparks, Research Biologist Rocky Mountain Bird Observatory 14500 Lark Bunting Lane Brighton, CO 80603 303.659.4348 www.rmbo.org ROCKY MOUNTAIN BIRD OBSERVATORY The mission of the Rocky Mountain Bird Observatory (RMBO) is to conserve birds of the Rocky Mountains, Great Plains, and Intermountain West and the habitats on which they depend through research, monitoring, education, and outreach. RMBO practices a multi- faceted approach to bird conservation that integrates scientific research and monitoring studies with education and outreach programs to bring bird conservation issues to the public and other conservation partners. RMBO works closely with state and federal natural resource agencies, private landowners, schools, and other nonprofit organizations. RMBO accomplishes its mission by working in four areas: Research : RMBO studies avian responses to habitat conditions, ecological processes, and management actions to provide scientific information that guides bird conservation actions. Monitoring : RMBO monitors the distribution and abundance of birds through long-term, broad-scale monitoring programs that track population trends for birds of the region. Education : RMBO provides active, experiential, education programs for K-12 students in order to create an awareness and appreciation for birds, with the goal of understanding the need for bird conservation. Outreach : RMBO shares the latest information in land management and bird conservation practices with private landowners, land managers, and resource professionals at natural resource agencies. -

Watchable Wildlife Form
U.S. Fish & Wildlife Service Watchable Wildlife Bitter Lake National Wildlife Refuge Welcome Roundnose minnow Dionda episcopa Amphibians Bitter Lake National Wildlife Refuge is one Speckled chub Extrarius aestivalis Family Ambystomatidae – Mole Salamanders of New Mexico’s most important sanctuaries Plains minnow Hybognathus placitus Tiger salamander Ambystoma tigrinum Arkansas River shiner Notropis girardi and breeding grounds for migratory birds and Family Leptodactylidae – Tropical Frogs Rio Grande shiner Notropis jemezanus other wildlife. Established in 1937, the 24,500- Eastern barking frog Eleutherodactylus augusti Pecos bluntnose shiner Notropis simus pecosensis acre refuge is strategically located along the latrans Pecos River where the Chihuahuan Desert Fathead minnow Pimephales promelas Family Pelobatidae – Spadefoot Toads meets the Great Plains. The convergence of Family Catostomidae – Suckers Couch’s spadefoot toad Scaphiopus couchii these vastly different terrains has produced a River carpsucker Carpoides carpio diverse range of habitats, providing a home to New Mexico spadefoot toad Spea multiplicata a rich array of plant and animal life, including Family Ictaluridae – Catfishes Plains spadefoot toad Spea bombifrons Channel catfish Ictalurus punctatus a number of rare species. Family Bufonidae – Toads Bitter Lake NWR is best known for its Family Cyprinodontidae – Pupfish Woodhouse’s toad Bufo woodhousii spectacular variety of birds, particularly the Pecos pupfish Cyprinodon pecosensis Red-spotted toad Bufo punctatus Great Plains toad Bufo cognatus large migrations of ducks, geese, and cranes Family Fundulidae – Killifishes Texas toad Bufo speciosus during the fall and winter months. Many of Plains killifish Fundulus zebrinus Western green toad Bufo debilis insidior these creatures are drawn by the refuge’s Rainwater killifish Lucania parva wetlands, which offer an abundance of food as Family Hylidae – Treefrogs Family Poeciliidae – Livebearers well as ideal nesting habitat for some species. -

A Study of the Genus Scaphiopus: the Spade-Foot Toads
Great Basin Naturalist Volume 1 Number 1 Article 4 7-25-1939 A study of the genus Scaphiopus: the spade-foot toads Vasco M. Tanner Brigham Young University, Provo, UT Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Tanner, Vasco M. (1939) "A study of the genus Scaphiopus: the spade-foot toads," Great Basin Naturalist: Vol. 1 : No. 1 , Article 4. Available at: https://scholarsarchive.byu.edu/gbn/vol1/iss1/4 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. A STUI)^' ( )F THE CiI':NUS SCAIMIIOPUS^^) Thk Spade-foot Toads AWSCO M. TANNER Professor of Zoology and Entomolog\- Brigham Young University INTRODUCTION It is a little more than a Inindred years since Holbrook, 1836, erected the t;enus Scaplilopits descril)in,ij solifariiis, a species found along the Atlantic Coast, as the type of the genus. This species, how- ever, was described the year prevousl}-, 1835, by Harlan as Rana Jiolhrookii : thus holhrookii becomes the accepted name of the genotype. Many species and sub-species have been named since this time, the great majority of them, however, have been considered as synonyms. In this study I liave recognized the following species: holhrookii, hitrtcrii, couchii, homhifrons, haminondii, and interttiontamis. A vari- ety of holhrookii, described by Garman as alhiis, from Key West. Florida, may be a vaUd form ; but since I have had only a specimen or two for study. -
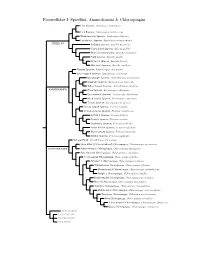
Passerellidae Species Tree
Passerellidae I: Spizellini, Ammodramini & Chlorospingini Lark Sparrow, Chondestes grammacus Lark Bunting, Calamospiza melanocorys Black-throated Sparrow, Amphispiza bilineata Five-striped Sparrow, Amphispiza quinquestriata SPIZELLINI Chipping Sparrow, Spizella passerina Clay-colored Sparrow, Spizella pallida Black-chinned Sparrow, Spizella atrogularis Field Sparrow, Spizella pusilla Brewer’s Sparrow, Spizella breweri Worthen’s Sparrow, Spizella wortheni Tumbes Sparrow, Rhynchospiza stolzmanni Stripe-capped Sparrow, Rhynchospiza strigiceps Grasshopper Sparrow, Ammodramus savannarum Grassland Sparrow, Ammodramus humeralis Yellow-browed Sparrow, Ammodramus aurifrons AMMODRAMINI Olive Sparrow, Arremonops rufivirgatus Green-backed Sparrow, Arremonops chloronotus Black-striped Sparrow, Arremonops conirostris Tocuyo Sparrow, Arremonops tocuyensis Rufous-winged Sparrow, Peucaea carpalis Cinnamon-tailed Sparrow, Peucaea sumichrasti Botteri’s Sparrow, Peucaea botterii Cassin’s Sparrow, Peucaea cassinii Bachman’s Sparrow, Peucaea aestivalis Stripe-headed Sparrow, Peucaea ruficauda Black-chested Sparrow, Peucaea humeralis Bridled Sparrow, Peucaea mystacalis Tanager Finch, Oreothraupis arremonops Short-billed (Yellow-whiskered) Chlorospingus, Chlorospingus parvirostris CHLOROSPINGINI Yellow-throated Chlorospingus, Chlorospingus flavigularis Ashy-throated Chlorospingus, Chlorospingus canigularis Sooty-capped Chlorospingus, Chlorospingus pileatus Wetmore’s Chlorospingus, Chlorospingus wetmorei White-fronted Chlorospingus, Chlorospingus albifrons Brown-headed