A New Species of Bird's Nest Fungi: Characterisation of <I>Cyathus Subglobisporus</I>
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Why Mushrooms Have Evolved to Be So Promiscuous: Insights from Evolutionary and Ecological Patterns
fungal biology reviews 29 (2015) 167e178 journal homepage: www.elsevier.com/locate/fbr Review Why mushrooms have evolved to be so promiscuous: Insights from evolutionary and ecological patterns Timothy Y. JAMES* Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, MI 48109, USA article info abstract Article history: Agaricomycetes, the mushrooms, are considered to have a promiscuous mating system, Received 27 May 2015 because most populations have a large number of mating types. This diversity of mating Received in revised form types ensures a high outcrossing efficiency, the probability of encountering a compatible 17 October 2015 mate when mating at random, because nearly every homokaryotic genotype is compatible Accepted 23 October 2015 with every other. Here I summarize the data from mating type surveys and genetic analysis of mating type loci and ask what evolutionary and ecological factors have promoted pro- Keywords: miscuity. Outcrossing efficiency is equally high in both bipolar and tetrapolar species Genomic conflict with a median value of 0.967 in Agaricomycetes. The sessile nature of the homokaryotic Homeodomain mycelium coupled with frequent long distance dispersal could account for selection favor- Outbreeding potential ing a high outcrossing efficiency as opportunities for choosing mates may be minimal. Pheromone receptor Consistent with a role of mating type in mediating cytoplasmic-nuclear genomic conflict, Agaricomycetes have evolved away from a haploid yeast phase towards hyphal fusions that display reciprocal nuclear migration after mating rather than cytoplasmic fusion. Importantly, the evolution of this mating behavior is precisely timed with the onset of diversification of mating type alleles at the pheromone/receptor mating type loci that are known to control reciprocal nuclear migration during mating. -

Revision of the Genus Cyathus (Basidiomycota) from the Herbaria of Northeast Brazil
Mycosphere 5 (4): 531–540 (2014) ISSN 2077 7019 www.mycosphere.org Article Mycosphere Copyright © 2014 Online Edition Doi 10.5943/mycosphere/5/4/5 Revision of the genus Cyathus (Basidiomycota) from the herbaria of northeast Brazil Cruz RHSF1, Assis NM2, Silva MA3 and Baseia IG4 1Programa de Pós-Graduação em Sistemática e Evolução, Centro de Biociências, Universidade Federal do Rio Grande do Norte, Avenida Senador Salgado Filho, 3000, Natal-RN 59.078-970 Brazil, [email protected] 2Departamento de Botânica e Zoologia, Centro de Biociências, Universidade Federal do Rio Grande do Norte, Avenida Senador Salgado Filho, 3000, Natal-RN 59.078-970 Brazil, [email protected] 3Departamento de Micologia, Universidade Federal de Pernambuco, Avenida Professor Moraes Rego 1235, Recife-PE 50.670-901 Brazil, [email protected] 4Departamento de Botânica e Zoologia, Centro de Biociências, Universidade Federal do Rio Grande do Norte, Avenida Senador Salgado Filho, 3000, Natal-RN 59.078-970 Brazil, [email protected] Cruz RHSF, Assis NM, Silva MA, Baseia IG 2014 – Revision of the genus Cyathus (Basidiomycota) from the herbaria of northeast Brazil. Mycosphere 5(4), 531−540, Doi 10.5943/mycosphere/5/4/5 Abstract Seventy exsiccates of the genus Cyathus deposited in JPB, UESC, URM and UFRN herbaria were studied and nine species were identified: Cyathus badius, C. berkeleyanus, C. earlei, C. gracilis, C. limbatus, C. pallidus, C. poeppigii, C. setosus and C. striatus. Cyathus berkeleyanus and C. poeppigii are recorded for the first time for northeastern Brazil. Descriptions, taxonomic remarks and illustrations of the studied material are presented. Key words – herbarium collection – Nidulariaceae – Gasteromycetes – taxonomic review Introduction The genus Cyathus Haller belongs to the family Nidulariaceae, included in the agaricoid clade of Basidiomycota (Matheny et al. -

Fluted Bird's Nest Fungus, Cyathus Striatus
A Horticulture Information article from the Wisconsin Master Gardener website, posted 19 Sept 2014 Fluted Bird’s Nest Fungus, Cyathus striatus There are many fungi in several genera called bird’s nest fungi because of the resemblance of their fruiting bodies to a tiny nest fi lled with eggs. One of the most common in Wisconsin is Cyathus striatus, the fl uted bird’s nest fungus. This species is widespread throughout temperate regions of the world, developing on dead wood in open forests, typically growing individually or in clusters on small twigs and fallen branches or other wood debris. Because it also grows readily in bark or wood mulch, it is frequently found in landscaped yards and gardens. Other species grow on plant remains or cow or horse dung. C. striatus, and others, are most commonly Fruiting bodies of fl uted bird’s nest seen in the autumn fungus, Cyathus striatus. when damp conditions promote their development, but they can be seen anytime conditions are appropriate. Even though each individual is small and inconspicuous, this species often grows in huge clusters, making A large cluster of fl uted bird’s nest fungi growing them more noticeable – on bark mulch. although they blend in so well with their background that it is very easy to overlook them. All of the bird’s nest fungi look like miniature nests (generally only ¼ inch in diameter) fi lled with four or fi ve tiny eggs. The cup-shaped “nest”, called a peridium, may be brown, gray or white, and smooth or textured inside and out. -

Growth and Antibacterial Metabolite Production by Wild Mushrooms
African Journal of Biomedical Research, Vol. 8 (2005); 157 - 162 ISSN 1119 – 5096 © Ibadan Biomedical Communications Group Available online at http://www.bioline.org.br/md Full Length Research Article Mycelial Growth and Antibacterial Metabolite Production by Wild Mushrooms 1*Shittu, O.B; 2Alofe, F.V; 3Onawunmi, G.O; 4Ogundaini, A.O; and Tiwalade, T.A.4 *1Department of Microbiology, University of Agriculture, Abeokuta and Departments of 2Microbiology, 3Pharmaceutical Microbiology and Pharmaceutical Chemistry, Obafemi Awolowo University, Ile-Ife, Received: May, 2005 Accepted: September, 2005 ABSTRACT Russula sp. and Pycnoporus cinnabarinus (wild mushrooms) were subjected to laboratory cultivation by spore germination and tissue culturing on Sabouraud dextrose agar plates. Subsequently, the growth and production of metabolite(s) were monitored in submerged fermentation for 7days using agar diffusion method. The result obtained showed that metabolite production peaked on the fourth day in Russula sp. and on the fifth day in Pycnoporus cinnabarinus with subsequent decrease in activity of the fermentation extract. Dry weight increases with fermentation time in both mushrooms. Keywords: Wild mushroom, Spore germination, Tissue culturing, Antibacterial metabolite INTRODUCTION Some mushrooms are used for the treatment of gastric ulcer, duodenal ulcer and chronic gastritis. Some mushrooms contain compounds, which can A good example is Hericium erinacius (Oei, 1991, make a contribution to the general health of man 1996). Some mushrooms such as Tremella (Elliot, 1997). As mushrooms are widely fulciformis are used for curing leukaemia, distributed all over the world, some of them have coughing, phlegm and asthma of patients suffering been used in traditional medicine as anti- from chronic bronchitis (Oei, 1991, 1996). -

Allescheria Boydii Shear
OBSERVATIONS ON THE MORPHOLCGY, PHYSIOLCGY, AND LIFE HISTORY OF ALLESCHERIA BOYDII SHEAR. by Robert Denton Flory A Thesis Presented to the Graduate School of the University of Richmond in Partial Fulfillment of the Requirements for the Degree of Master of Arts University of Richmond June, 1956 LlBRARY UNIVERSITY OF RICHMOND VIRGINIA ACKNOWLEDGEMENTS To those individuals who have contributed to the success of this thesis, I wish to express rrry sincere appreciation. I am especially grateful to Dr. R. F. Smart, who made possible for me the opportunity to study at the University of Richmond, and for his constant guidance throughout the preparation of this thesis. I am also greatly indebted to the other members of the faculty at the University of Richmond Biology Department for their advice and for the many considerations they have shown. It is a pleasure to acknowledge the aid of Dr. C. W. Emmons of the National Institute of Health for making available many strains of the fungus studied. Grateful acknowledgement is made to Dr. R. F. Smart, Dr •. J. D. Burke, Mr. A.H. O'Bier, Jr. and Mr. R. F. Moore,Jr,. for their assistance with the photographic plates of this thesis. LIBRARY °lJNlVERSlTY OF RICHMOND VIRGINIA II CONTENTS page ACl<l'iOWI.EDG~S. • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • II IN'rRODUCTI ON •• ·• • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 1 ~I.AI.iS .AND J.i:E'rHODS. • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 3 RESULTS OF MORPHOLOGIC.AL .AND CYTOLOGICAL STUDY ••••••••••• 6 Colony Characteristics ••••••••••••••.••••••••••••••• 6 Vegetative Hyphae •••.•.•••••.••••.••.•••.••••••••••. 8 Asexual Reprod.uction. • . • . • • . • • • • • . • • • • • . • • • • • • • • . • • l2 Sexual Reproduction ••••.•••••••••••••••• 18 PHYSIC.AL EFFECTS OF ENVIRONMENT UPON GROWTH. • . • • • • • • • • • • • 28 NUTRITIONAL STUDIES .AND OCCURRENCE IN NATURE •••••..•••.•• 32 LIFE HISTORY, SEXUALITY, .AND TAXONOMY Life History •• . -
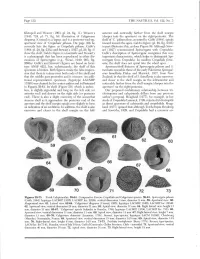
(1943: 724, Pi. 71, Fig. 16) Illustration of Calyptraea (Deeper Into the Aperture) on the Right/Posterior
Page 118 THE NAUTILUS, Vol. 122, No. 3 Kleinpeil and Weaver (1963, pi. 24, fig. 11). Weavers anterior and noticeably farther from the shell margin (1943: 724, pi. 71, fig. 16) illustration of Calyptraea (deeper into the aperture) on the right/posterior. The diegoana (Conrad) is a lapsus and is a posterior-end-up, shelf of 'C.y pileum thus, as noted by Gabb (1864), spirals apertural view of 'Crepidula' pileum. On page 356 he inward toward the apex. Gabb's figure (pi. 29, fig. 233b) correctly lists the figure as Crepidula pileum. Gabb's in part illustrates this, as does Figure 59. Although Stew- (1864: pi. 29, fig. 233a) and Stewart's (1927, pi. 29, fig. 3) art (1927) synonomized Spirocrypta with Crepidula, show the shelf. Gabb's figure is a fascimile and Stewart's Gabb's description of Spirocrypta recognizes this very is a photograph that has been reproduced in other dis- important characteristic, which helps to distinguish Spi- cussions of Spirocrypta (e.g., Wenz, 1940: 903, fig. rocrypta from Crepidula. In modern Crepidula forni- 2660a). Gabb's and Stewart's figures are based on lecto- cata, the shelf does not spiral into the whorl apex. type ANSP 4221, but, unfortunately, the shelf of this Aperture/shelf features of Spirocrypta pileum and S. specimen is broken. Both figures create the false impres- inomata resemble those of the early Paleocene Spirogal- sion that there is a sinus near both ends of the shelf and erus lamellaria Finlay and Marwick, 1937, from New that the middle part protrudes and is concave. -

<I>Nidula Shingbaensis</I>
ISSN (print) 0093-4666 © 2013. Mycotaxon, Ltd. ISSN (online) 2154-8889 MYCOTAXON http://dx.doi.org/10.5248/125.53 Volume 125, pp. 53–58 July–September 2013 Nidula shingbaensis sp. nov., a new bird’s nest fungus from India Kanad Das 1 & Rui Lin Zhao 2* 1Botanical Survey of India, SHRC, Gangtok 737103, Sikkim, India 2 Key Laboratory of Forest Disaster Warning and Control in Yunnan Province, Southwest Forestry University, Kunming, Yunnan Prov. 650224, PR China * Correspondence to: [email protected] Abstract —A new species of bird’s nest fungi, Nidula shingbaensis, is proposed from the state of Sikkim. It is characterised by a slightly flared moderate to large peridium, yellowish interior peridium-wall, numerous brown-coloured peridioles with irregularly wrinkled surfaces, large broadly ellipsoid to elongate basidiospores, and a six-layered (in cross- section) peridium. A detailed description is supported by macro- and micromorphological illustrations, and the relation with similar and related taxa is discussed. Key words — Basidiomycota, macrofungi, Agaricaceae, Agaricales, taxonomy Introduction Bird’s nest fungi, previously placed in a separate family Nidulariaceae, were recently moved to the Agaricaceae (Kirk et al. 2008). Currently, they are represented in India by three genera with 17 species (14 Cyathus spp., Nidula emodensis, N. candida, and one Crucibulum sp.; Das & Zhao 2012). Shingba Rhododendron Sanctuary (43 km2) lies in the North district of Sikkim (a small Indian state in the eastern Himalaya). This subalpine area in the Yumthang valley and surroundings is covered by over 40 Rhododendron species but otherwise dominated by trees (Abies densa, Picea spinulosa, Tsuga dumosa, Larix griffithii, Magnolia globosa, M. -

Bivalves and Gastropods of the Gulf of Tehuantepec, Mexico: a Checklist of Species with Notes on Their Habitat and Local Distribution
Hindawi Publishing Corporation Journal of Marine Biology Volume 2009, Article ID 176801, 12 pages doi:10.1155/2009/176801 Research Article Bivalves and Gastropods of the Gulf of Tehuantepec, Mexico: A Checklist of Species with Notes on Their Habitat and Local Distribution Eduardo Rıos-Jara,´ Ceciel-M. Navarro-Caravantes, Cristian-M. Galvan-Villa,´ and Ernesto Lopez-Uriarte Laboratorio de Ecosistemas Marinos y Acuicultura, Departamento de Ecolog´ıa, Centro Universitario de Ciencias Biologicas´ y Agropecuarias, Universidad de Guadalajara, Carretera a Nogales Km. 15.5, Las Agujas Nextipac, Zapopan, Jalisco 45110, Mexico Correspondence should be addressed to Eduardo R´ıos-Jara, [email protected] Received 1 April 2009; Accepted 19 October 2009 Recommended by Ricardo Serrao˜ Santos The taxonomic composition of 160 species of bivalves and gastropods recorded in the Gulf of Tehuantepec is presented with information on their habitat and distribution along 10 different localities of the shoreline and 42 stations of the continental shelf. The species were on sandy and rocky beaches, coastal lagoons, estuaries, mangroves, rocky breakwaters of ports, and shallow subtidal areas (14–47 m depth). A total of 78 bivalve species and 82 gastropod species were recorded. Most of these were associated with sandy and rocky beaches and breakwaters of ports. The estuaries host 30 species and the coastal lagoons only two. In the shallow subtidal there were 18 gastropod species and 40 bivalve species representing 36.3% of all. This study adds 24 bivalve species and 29 gastropod species not recorded in previous studies for a total count of 213 species (102 bivalves and 111 gastropods) for Gulf of Tehuantepec. -

Megaphylogeny Resolves Global Patterns of Mushroom Evolution
Lawrence Berkeley National Laboratory Recent Work Title Megaphylogeny resolves global patterns of mushroom evolution. Permalink https://escholarship.org/uc/item/0tx3k9hc Journal Nature ecology & evolution, 3(4) ISSN 2397-334X Authors Varga, Torda Krizsán, Krisztina Földi, Csenge et al. Publication Date 2019-04-01 DOI 10.1038/s41559-019-0834-1 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Europe PMC Funders Group Author Manuscript Nat Ecol Evol. Author manuscript; available in PMC 2019 September 18. Published in final edited form as: Nat Ecol Evol. 2019 April ; 3(4): 668–678. doi:10.1038/s41559-019-0834-1. Europe PMC Funders Author Manuscripts Megaphylogeny resolves global patterns of mushroom evolution A full list of authors and affiliations appears at the end of the article. Abstract Mushroom-forming fungi (Agaricomycetes) have the greatest morphological diversity and complexity of any group of fungi. They have radiated into most niches and fulfill diverse roles in the ecosystem, including wood decomposers, pathogens or mycorrhizal mutualists. Despite the importance of mushroom-forming fungi, large-scale patterns of their evolutionary history are poorly known, in part due to the lack of a comprehensive and dated molecular phylogeny. Here, using multigene and genome-based data, we assemble a 5,284-species phylogenetic tree and infer ages and broad patterns of speciation/extinction and morphological innovation in mushroom- forming fungi. Agaricomycetes started a rapid class-wide radiation in the Jurassic, coinciding with the spread of (sub)tropical coniferous forests and a warming climate. A possible mass extinction, several clade-specific adaptive radiations, and morphological diversification of fruiting bodies followed during the Cretaceous and the Paleogene, convergently giving rise to the classic toadstool morphology, with a cap, stalk, and gills (pileate-stipitate morphology). -

The Fascinating Bird's Nest Mushroom, Secondary Metabolites And
International Journal of Pharma Research and Health Sciences, 2021; 9 (1): 3265-3269 DOI:10.21276/ijprhs.2021.01.01 Waill and Ghoson CODEN (USA)-IJPRUR, e-ISSN: 2348-6465 Mini Review The Fascinating Bird’s Nest Mushroom, Secondary Metabolites and Biological Activities Waill A Elkhateeb*, Ghoson M Daba Chemistry of Natural and Microbial Products Department, Pharmaceutical Industries Division, National Research Centre, Dokki, Giza, 12622, Egypt. ARTICLE INFO: ABSTRACT: Received: 05 Feb 2021 Background: Mushrooms are generous source of nutritional and medicinal compounds. Accepted: 16 Feb 2021 Bird’s nest fungi are a gasteromyceteous group of mushrooms named for their similarity in Published: 28 Feb 2021 shape to small bird’s nests. They are considered from the tiniest and most interesting mushrooms all over the world. It is usually found in shady moist environments, and typically survive on plant debris, soil, decaying wood, or animal’s excrement. Bird’s nest mushrooms Corresponding author * are inedible, though they were not previously reported to be poisonous, due to their tiny size. Waill A Elkhateeb, Object: this review aims to put bird’s nest mushrooms under light spot through describing Chemistry of Natural and their morphology and ecology especially of the most common fungus, Cyathus haller. Microbial Products Department, Moreover, discussing important secondary metabolites and biological activities exerted by Pharmaceutical Industries bird’s nest mushrooms. Division, National Research Conclusion: bird’s nest mushrooms are able to produce many novel and potent secondary Centre, Dokki, Giza, 12622, metabolites that exerted different bioactivities especially as antimicrobial, antitumor, and Egypt. anti-neuro inflammation activities. Further studies and investigations are encouraged in E Mail: [email protected] order to find more about this interesting tiny mushroom. -

Britt A. Bunyard Onto the Scene Around 245 MYA
Figure 1. Palaeoagaracites antiquus from Burmese amber, 100 MYA. This is the oldest-known fossilized mushroom. Photo courtesy G. Poinar. Britt A. Bunyard onto the scene around 245 MYA. Much of what we know of extinct ow old are the oldest fungi? fungi comes from specimens found How far back into the geological in amber. Amber is one medium that record do fungi go … and how preserves delicate objects, such as fungal Hdo we know this? They surely do not bodies, in exquisite detail (Poinar, 2016). fossilize, right? This is due to the preservative qualities It turns out that although soft fleshy of the resin when contact is made with fungi do not fossilize very well, we do entrapped plants and animals. Not only have a fossil record for them. (Indeed, I does the resin restrict air from reaching can recommend an excellent book, Fossil the fossils, it withdraws moisture from Fungi by Taylor et al.; 2014; Academic the tissue, resulting in a process known Press.) The first fungi undoubtedly as inert dehydration. Furthermore, originated in water; estimates of their age amber not only inhibits growth of mostly come from “molecular clocks” microbes that would decay organic and not so much from fossils. Based on matter, it also has properties that kill fossil record, fungi are presumed to have microbes. Antimicrobial compounds in Figure 2. Coprinites dominicana from been present in Late Proterozoic (900- the resin destroy microorganisms and Dominican amber, 20 MYA. Photo 570 MYA) (Berbee and Taylor, 1993). “fix” the tissues, naturally embalming courtesy G. Poinar. The oldest “fungus” microfossils were anything that gets trapped there by a found in Victoria Island shale and date to process of polymerization and cross- around 850M-1.4B years old (Butterfield, bonding of the resin molecules (Poinar 2005), though the jury is still out on if and Hess, 1985). -

Fungi of the Iowa Loess Hills
Proceedings of the Iowa Academy of Science Volume 92 Number Article 7 1985 Fungi of the Iowa Loess Hills L. H. Tiffany Iowa State University J. F. Shearer Iowa State University A. W. Gabel Iowa State University G. Knaphus Iowa State University Let us know how access to this document benefits ouy Copyright © Copyright 1985 by the Iowa Academy of Science Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Tiffany, L. H.; Shearer, J. F.; Gabel, A. W.; and Knaphus, G. (1985) "Fungi of the Iowa Loess Hills," Proceedings of the Iowa Academy of Science, 92(5), 180-185. Available at: https://scholarworks.uni.edu/pias/vol92/iss5/7 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Tiffany et al.: Fungi of the Iowa Loess Hills Proc. Iowa Acad. Sci. 92(5):180-185, 1985 Fungi of the Iowa Loess Hills L. H. TIFFANY,). F. SHEARER, A. W. GABEL, and G. KNAPHUS Department of Botany, Iowa State University, Ames, IA 50011 From 1981 through 1983 biological surveys of the Loess Hills were spoonsored by the Iowa State Preserves Advisory Board. Twenty-four sites in 7 counties were visited during the first week in June during these years. Collections were made of all macrofungi producing identifiable fruiting structures or represented by recognizable weathered fruiting structures developed the previous fall.