Changes in Morphology and Function of Adrenal Cortex in Mice Fed a High-Fat Diet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hedgehog Signaling Is Evolutionarily Conserved Cilium-Independent
Downloaded from genesdev.cshlp.org on August 14, 2009 - Published by Cold Spring Harbor Laboratory Press Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved Miao-Hsueh Chen, Christopher W. Wilson, Ya-Jun Li, et al. Genes Dev. 2009 23: 1910-1928 Access the most recent version at doi:10.1101/gad.1794109 Supplemental http://genesdev.cshlp.org/content/suppl/2009/07/23/23.16.1910.DC1.html Material References This article cites 97 articles, 47 of which can be accessed free at: http://genesdev.cshlp.org/content/23/16/1910.full.html#ref-list-1 Email alerting Receive free email alerts when new articles cite this article - sign up in the box at the service top right corner of the article or click here To subscribe to Genes & Development go to: http://genesdev.cshlp.org/subscriptions Copyright © 2009 by Cold Spring Harbor Laboratory Press Downloaded from genesdev.cshlp.org on August 14, 2009 - Published by Cold Spring Harbor Laboratory Press Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved Miao-Hsueh Chen,1,3 Christopher W. Wilson,1,3 Ya-Jun Li,1 Kelvin King Lo Law,2 Chi-Sheng Lu,1 Rhodora Gacayan,1 Xiaoyun Zhang,2 Chi-chung Hui,2 and Pao-Tien Chuang1,4 1Cardiovascular Research Institute, University of California at San Francisco, San Francisco, California 94158, USA; 2Program in Developmental and Stem Cell Biology, The Hospital for Sick Children, and Department of Molecular Genetics, University of Toronto, Toronto, Ontario M5G 1L7, Canada A central question in Hedgehog (Hh) signaling is how evolutionarily conserved components of the pathway might use the primary cilium in mammals but not fly. -

GLI1-Amplifications Expand the Spectrum of Soft Tissue Neoplasms Defined by GLI1 Gene Fusions
Modern Pathology (2019) 32:1617–1626 https://doi.org/10.1038/s41379-019-0293-x ARTICLE GLI1-amplifications expand the spectrum of soft tissue neoplasms defined by GLI1 gene fusions 1 1 1 2 3 Narasimhan P. Agaram ● Lei Zhang ● Yun-Shao Sung ● Samuel Singer ● Todd Stevens ● 3 4 5 6 6 Carlos N. Prieto-Granada ● Justin A. Bishop ● Benjamin A. Wood ● David Swanson ● Brendan C. Dickson ● Cristina R. Antonescu1 Received: 18 February 2019 / Revised: 29 April 2019 / Accepted: 1 May 2019 / Published online: 12 June 2019 © United States & Canadian Academy of Pathology 2019 Abstract GLI1 fusions involving ACTB, MALAT1, and PTCH1 genes have been recently reported in a subset of malignant soft tissue tumors with characteristic monomorphic nested epithelioid morphology and frequent S100 positivity. However, we encountered a group of morphologically similar soft tissue tumors lacking the canonical GLI1 gene fusions and sought to investigate their genetic abnormalities. A combined approach including RNA sequencing, targeted exome sequencing and FISH methodologies were used to identify potential novel genetic abnormalities. Ten patients (five females, five males) with – fi 1234567890();,: 1234567890();,: an age range of 4 65 years (median 32.5) were identi ed. Tumors were located in the soft tissues of the limbs, trunk and head and neck, with one each in the tongue and lung. Histologically, tumors revealed ovoid to epithelioid cells arranged in a distinctive nested-trabecular pattern, separated by thin septa and a delicate vascular network. Two cases showed areas of increased nuclear pleomorphism and focal fascicular spindle cell growth. Four tumors showed a high mitotic count (≥15/10 HPFs), with necrosis seen in three of them. -

Expression Pattern of Delta-Like 1 Homolog in Developing Sympathetic Neurons and Chromaffin Cells
Published in "Gene Expression Patterns 30: 49–54, 2018" which should be cited to refer to this work. Expression pattern of delta-like 1 homolog in developing sympathetic neurons and chromaffin cells ∗ Tehani El Faitwria,b, Katrin Hubera,c, a Institute of Anatomy & Cell Biology, Albert-Ludwigs-University Freiburg, Albert-Str. 17, 79104, Freiburg, Germany b Department of Histology and Anatomy, Faculty of Medicine, Benghazi University, Benghazi, Libya c Department of Medicine, University of Fribourg, Route Albert-Gockel 1, 1700, Fribourg, Switzerland ABSTRACT Keywords: Delta-like 1 homolog (DLK1) is a member of the epidermal growth factor (EGF)-like family and an atypical notch Sympathetic neurons ligand that is widely expressed during early mammalian development with putative functions in the regulation Chromaffin cells of cell differentiation and proliferation. During later stages of development, DLK1 is downregulated and becomes DLK1 increasingly restricted to specific cell types, including several types of endocrine cells. DLK1 has been linked to Adrenal gland various tumors and associated with tumor stem cell features. Sympathoadrenal precursors are neural crest de- Organ of Zuckerkandl rived cells that give rise to either sympathetic neurons of the autonomic nervous system or the endocrine Development ffi Neural crest chroma n cells located in the adrenal medulla or extraadrenal positions. As these cells are the putative cellular Phox2B origin of neuroblastoma, one of the most common malignant tumors in early childhood, their molecular char- acterization is of high clinical importance. In this study we have examined the precise spatiotemporal expression of DLK1 in developing sympathoadrenal cells. We show that DLK1 mRNA is highly expressed in early sympa- thetic neuron progenitors and that its expression depends on the presence of Phox2B. -

Non-Canonical Activation of Hedgehog in Prostate Cancer Cells Mediated by the Interaction of Transcriptionally Active Androgen Receptor Proteins with Gli3
Oncogene (2018) 37:2313–2325 https://doi.org/10.1038/s41388-017-0098-7 ARTICLE Non-canonical activation of hedgehog in prostate cancer cells mediated by the interaction of transcriptionally active androgen receptor proteins with Gli3 1 1,2 2 1 1 1 2,3 Na Li ● Sarah Truong ● Mannan Nouri ● Jackson Moore ● Nader Al Nakouzi ● Amy Anne Lubik ● Ralph Buttyan Received: 19 July 2017 / Revised: 18 October 2017 / Accepted: 29 November 2017 / Published online: 12 February 2018 © The Author(s) 2018. This article is published with open access Abstract Hedgehog (Hh) is an oncogenic signaling pathway that regulates the activity of Gli transcription factors. Canonical Hh is a Smoothened-(Smo-) driven process that alters the post-translational processing of Gli2/Gli3 proteins. Though evidence supports a role for Gli action in prostate cancer (PCa) cell growth and progression, there is little indication that Smo is involved. Here we describe a non-canonical means for activation of Gli transcription in PCa cells mediated by the binding of transcriptionally-active androgen receptors (ARs) to Gli3. Androgens stimulated reporter expression from a Gli-dependent promoter in a variety of AR + PCa cells and this activity was suppressed by an anti-androgen, Enz, or by AR knockdown. 1234567890();,: Androgens also upregulated expression of endogenous Gli-dependent genes. This activity was associated with increased intranuclear binding of Gli3 to AR that was antagonized by Enz. Fine mapping of the AR binding domain on Gli2 showed that AR recognizes the Gli protein processing domain (PPD) in the C-terminus. Mutations in the arginine-/serine repeat elements of the Gli2 PPD involved in phosphorylation and ubiquitinylation blocked the binding to AR. -

I. Introduction B. Adrenal Cortex
I. Introduction Adrenal Glands • suprarenal – they sit on top of the kidneys • each is composed of 2 distinct regions: A. Adrenal Medulla - the inner region - comprises 20% of the gland - secretes epinephrine and norepinephrine - derived from ectoderm B. Adrenal Cortex 1) Zona Glomerulosa (outermost region) - produces mineralocorticoids (aldosterone) • the outer region 2) Zona Fasiculata (middle region) - produces glucocorticoids (cortisol) as well as • comprises 80% of the gland estrogens and androgens • secretes corticosteroids 3) Zona Reticularis (innermost region) • derived from mesoderm - same function as zona fasiculata DHEA – dehydroepiandrosterone • an adrenal androgen in females • responsible for growth of pubic and axillary hair C. Pathologies Associated with Adrenal II. Mineralocorticoids (Aldosterone) Androgen Hypersecretion A. Functions 1.Adrenogenital Syndrome - promotes reabsorption of Na+ and - hypersecretion of androgens or estrogens secretion of K+ from the distal portion of the a) in the adult female: nephron..primary regulator of salt balance and extracellular volume - masculinization (i.e. hirsutism) -Similar (but less important) effect on salt b) in the female embryo: transport in colon, salivary glands, and - female pseudohermaphroditism sweat glands. c) in the adult male: - no effect d) in young boys: - precocious pseudopuberty 1 II. Mineralocorticoids (Aldosterone) C. Pathologies B. Regulation of Secretion 1. Hypersecretion 1. Renin Angiotensin a. primary hyperaldosteronism - Angiotensin II stimulates aldost. secretion - Conn’s syndrome 2. Potassium - usually due to a tumor on the gland + - high levels of K induce aldost. secretion - too much secretion of gland itself 3. ACTH –no direct role b. secondary hyperaldosteronism - default in renin angiotensin system - most common in atherosclerosis of renal arteries C. Pathologies III. Glucocorticoids (Cortisol) 1. -
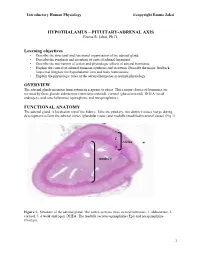
HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Learning Objectives OVERVIEW FUNCTIONAL ANATOMY
Introductory Human Physiology ©copyright Emma Jakoi HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Emma R. Jakoi, Ph.D. Learning objectives • Describe the structural and functional organization of the adrenal gland. • Describe the synthesis and secretion of cortical adrenal hormones. • Describe the mechanism of action and physiologic effects of adrenal hormones. • Explain the control of adrenal hormone synthesis and secretion. Describe the major feedback loops that integrate the hypothalamic axis and body homeostasis. • Explain the physiologic roles of the adrenal hormones in normal physiology. OVERVIEW The adrenal glands maintain homeostasis in response to stress. Three major classes of hormones are secreted by these glands: aldosterone (mineralocorticoid), cortisol (glucocorticoid), DHEA (weak androgen), and catecholamines (epinephrine and norepinephrine). FUNCTIONAL ANATOMY The adrenal gland is located on top of the kidney. Like the pituitary, two distinct tissues merge during development to form the adrenal cortex (glandular tissue) and medulla (modified neuronal tissue) (Fig 1). 1 2 cortex 3 medulla Figure 1. Structure of the adrenal gland. The cortex secretes three steroid hormones: 1. aldosterone, 2. cortisol, 3. a weak androgen, DHEA. The medulla secretes epinephrine (Epi) and norepinephrine (NorEpi). 1 Introductory Human Physiology ©copyright Emma Jakoi MINERALOCORTICOIDS The major mineralocorticoid in humans is aldosterone. Aldosterone is NOT under the hypothalamus- pituitary control and does not mediate a negative feedback to this axis. Aldosterone secretion is increased by the vasoconstrictor, angiotensin II, and by elevated plasma K+ concentration. Elevated plasma Na+ inhibits the secretion of aldosterone. Aldosterone, acts in the kidney to promote secretion of K+ into the urine from the blood and the reabsorption of Na+ from the urine into the blood. -

Dream Recall/Affect and the Hypothalamic–Pituitary–Adrenal Axis
Review Dream Recall/Affect and the Hypothalamic–Pituitary–Adrenal Axis Athanasios Tselebis 1, Emmanouil Zoumakis 2 and Ioannis Ilias 3,* 1 Department of Psychiatry, Sotiria Hospital, 115 27 Athens, Greece; [email protected] 2 First Department of Pediatrics, National and Kapodistrian University of Athens Medical School, Aghia Sophia Children’s Hospital, 115 27 Athens, Greece; [email protected] 3 Department of Endocrinology, Diabetes and Metabolism, Elena Venizelou Hospital, 115 21 Athens, Greece * Correspondence: [email protected]; Tel.: +30-213-205-1389 Abstract: In this concise review, we present an overview of research on dream recall/affect and of the hypothalamic–pituitary–adrenal (HPA) axis, discussing caveats regarding the action of hormones of the HPA axis (mainly cortisol and its free form, cortisol-binding globulin and glucocorticoid receptors). We present results of studies regarding dream recall/affect and the HPA axis under physiological (such as waking) or pathological conditions (such as in Cushing’s syndrome or stressful situations). Finally, we try to integrate the effect of the current COVID-19 situation with dream recall/affect vis-à-vis the HPA axis. Keywords: dreams; cortisol; stress; memory; sleep; HPA axis 1. Introduction—Dream Recall Almost all humans dream (indeed, there may be 0.5% of people who do not do so) [1]. Dreams are a series of images, thoughts and senses and are considered a specific type Citation: Tselebis, A.; Zoumakis, E.; of experience that occurs in our brain during sleep. During the dream, there is limited Ilias, I. Dream Recall/Affect and the control of the dream content, visual images and memory activation. -

The Adrenal Androgen Precursors DHEA and Androstenedione (A4)
PERIPHERAL METABOLISM OF THE ADRENAL STEROID 11Β- HYDROXYANDROSTENEDIONE YIELDS THE POTENT ANDROGENS 11KETO- TESTOSTERONE AND 11KETO-DIHYDROTESTOSTERONE Elzette Pretorius1, Donita J Africander1, Maré Vlok2, Meghan S Perkins1, Jonathan Quanson1 and Karl-Heinz Storbeck1 1University of Stellenbosch, Department of Biochemistry, Stellenbosch, South Africa 2University of Stellenbosch, Mass Spectrometry Unit, Stellenbosch, South Africa The adrenal androgen precursors DHEA and androstenedione (A4) play an important role in the development and progression of castration resistant prostate cancer (CRPC) as they are converted to dihydrotestosterone (DHT) by steroidogenic enzymes expressed in CRPC tissue. We have recently shown that the adrenal C19 steroid 11β-hydroxyandrostenedione (11OHA4) serves as a precursor to the androgens, 11-ketotestosterone (11KT) and 11-keto-5α-dihydrotestosterone (11KDHT), and that the latter two steroids could play a role in CRPC. The aim of this study was therefore to characterise 11KT and 11KDHT in terms of their androgenic activity. Competitive whole cell binding assays revealed that 11KT and 11KDHT bind to the human androgen receptor (AR) with affinities similar to that of testosterone (T) and DHT. Transactivation assays on a synthetic androgen response element (ARE) demonstrated that the potencies and efficacies of 11KT and 11KDHT are comparable to that of T and DHT, respectively. Moreover, we show that both 11KT and 11KDHT induce the expression of AR-regulated genes (KLK3, TMPRSS2 and FKBP5) and cellular proliferation in the androgen dependent prostate cancer cell lines, LNCaP and VCaP. In most cases, 11KT and 11KDHT upregulated AR-regulated gene expression and increased LNCaP cell growth to a significantly higher extent than T and DHT. Mass spectrometry-based proteomics revealed that 11KT and 11KDHT, like T and DHT, results in the upregulation of multiple AR-regulated proteins in VCaP cells, with 11KDHT regulating more AR-regulated proteins than DHT. -

The Adrenal Capsule Is a Signaling Center Controlling Cell Renewal and Zonation Through Rspo3
Downloaded from genesdev.cshlp.org on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press RESEARCH COMMUNICATION The permanent cortex is formed through recruitment of The adrenal capsule is a capsular cells in a process that involves SHH signaling signaling center controlling (King et al. 2009). By E17.5, steroidogenic cells have adopted specific expression profiles, with the outermost cell renewal and zonation cell layers (zona glomerulosa [ZG]) producing enzymes Rspo3 that are required for mineralocorticoid production (e.g., through CYP11B2), and deeper layers (zona fasciculata [ZF]) Valerie Vidal,1,2,3,9 Sonia Sacco,1,2,3,9 expressing genes involved in glucocorticoid synthesis Ana Sofia Rocha,1,2,3,8 Fabio da Silva,1,2,3 (Cyp11b1). In humans, but not rodents, a third layer (zona reticularis) can be distinguished that produces an- Clara Panzolini,1,2,3 Typhanie Dumontet,4,5 1,2,3 6 drogens and is located close to the medulla. Several lines Thi Mai Phuong Doan, Jingdong Shan, of evidence suggest that β-catenin plays an important Aleksandra Rak-Raszewska,6 Tom Bird,7 role in adrenal zonation and maintenance. Activation of Seppo Vainio,6 Antoine Martinez,4,5 the β-catenin pathway is restricted to the ZG (Kim et al. and Andreas Schedl1,2,3 2008; Walczak et al. 2014), and ectopic expression leads to the activation of ZG markers in ZF cells (Berthon 1Institute of Biology Valrose, Université de Nice-Sophia, F-06108 et al. 2010). Moreover, β-catenin seems to bind to and con- Nice, France; 2UMR1091, Institut National de la Santé et de la trol the expression of At1r, a gene specifically expressed Recherche Médicale, F-06108 Nice, France; 3CNRS, UMR7277, within the ZG (Berthon et al. -

Pig Gonads, Adrenal Glands and Brain C
Immunoreactive cytochrome P-45017\g=a\in rat and guinea- pig gonads, adrenal glands and brain C. Le Goascogne1, N. Sanan\l=e'\s1, M. Gou\l=e'\zou1, S. Takemori2, S. Kominami2, E. E. Baulieu1 and P. Robel1 1INSERM U33, Communications Hormonales, and Faculté de Médecine, Université Paris-sud, Lab Hormones F-94275 Bicêtre Cedex France 2 Faculty of Integrated Arts and Sciences, Hiroshima University, Hiroshima 730, Japan Summary. The cytochrome P-45017\g=a\-hydroxylase, 17\ar=r\20lyase (P-45017\g=a\) is the key enzyme responsible for the biosynthesis of androgens in steroidogenic organs. Its cellular localization has been examined with an immunohistochemical technique. In immature rat ovary, P-45017\g=a\was first detected in sparse interstitial cells on postnatal Day 8. The number of immunoreactive interstitial cells increased thereafter and the intensity of P-45017\g=a\staining in these cells was highest at 3 weeks of age. The intensity of staining then started to decline and was very faint at Day 35. From 6 weeks on, the distribution of immunoreactive P-45017\g=a\was of the adult type: it was detected exclusively in the thecal cells of the large antral, preovulatory, follicles. P-45017\g=a\was not detectable during pregnancy except on the day of parturition, when thecal cells were transiently immunoreactive. The staining had vanished 24 h after delivery. Human chorionic gonadotrophin (hCG), injected into immature females on Days 24 to 26, induced P-45017\g=a\prematurely in thecal cells. When injected on Days 12 to 14 of pregnancy, hCG also induced P-45017\g=a\in the thecal cells surrounding the largest follicles, whereas the interstitial and luteal cells were not immunostained. -

Adrenal Gland Hormones
CHAPTER 8 Adrenal Gland Hormones Devra K. Dang, PharmD, BCPS, CDE, FNAP | Trinh Pham, PharmD, BCOP | Jennifer J. Lee, PharmD, BCPS, CDE LEARNING OBJECTIVES KEY TERMS AND DEFINITIONS After completing this chapter, you should be able to ACTH (adrenocorticotropic hormone) — a hormone produced 1. Identify the hormones produced by the adrenal glands by the pituitary gland that stimulates 2. Describe the functions of mineralocorticoids and glucocorticoids in the body the adrenal cortex to produce glucocorticoids, mineralocorticoids, 3. Recognize the signs and symptoms of adrenal insuffi ciency and androgens. PART 4. Describe the pharmacological treatment of patients with acute and chronic adrenal Addison ’ s disease — a disorder insuffi ciency in which the adrenal glands do not produce enough steroid hormones. 3 5. Recognize the signs and symptoms of Cushing ’ s syndrome and the result of too Adenoma — a benign much cortisol (noncancerous) tumor of glandular 6. Describe the pharmacologic and nonpharmacologic management of patients with origin. Cushing ’ s syndrome Adrenal insuffi ciency — a term 7. List management strategies for administration of glucocorticoid and mineralocorti- referring to a defi ciency in the levels of adrenal hormones. coid therapy to avoid development of adrenal disorders Aldosterone — the hormone produced by the adrenal glands that regulates the balance of sodium, he adrenal glands are an integral part of the endocrine system, secreting water, and potassium concentrations in the body. T hormones that act throughout the body to regulate functions and promote Corticotropin-releasing homeostasis. In addition to the neurotransmitters epinephrine and norepineph- hormone (CRH) — a hormone rine, the corticosteroids secreted by the adrenal glands are vital to a wide released by the hypothalamus that variety of physiological processes. -

GLI2 but Not GLI1/GLI3 Plays a Central Role in the Induction of Malignant Phenotype of Gallbladder Cancer
ONCOLOGY REPORTS 45: 997-1010, 2021 GLI2 but not GLI1/GLI3 plays a central role in the induction of malignant phenotype of gallbladder cancer SHU ICHIMIYA1, HIDEYA ONISHI1, SHINJIRO NAGAO1, SATOKO KOGA1, KUKIKO SAKIHAMA2, KAZUNORI NAKAYAMA1, AKIKO FUJIMURA3, YASUHIRO OYAMA4, AKIRA IMAIZUMI1, YOSHINAO ODA2 and MASAFUMI NAKAMURA4 Departments of 1Cancer Therapy and Research, 2Anatomical Pathology, 3Otorhinolaryngology and 4Surgery and Oncology, Graduate School of Medical Sciences, Kyushu University, Fukuoka 812‑8582, Japan Received August 10, 2020; Accepted December 7, 2020 DOI: 10.3892/or.2021.7947 Abstract. We previously reported that Hedgehog (Hh) signal Introduction was enhanced in gallbladder cancer (GBC) and was involved in the induction of malignant phenotype of GBC. In recent Gallbladder cancer (GBC) is the seventh most common gastro- years, therapeutics that target Hh signaling have focused on intestinal carcinoma and accounts for 1.2% of all cancer cases molecules downstream of smoothened (SMO). The three tran- and 1.7% of all cancer-related deaths (1). GBC develops from scription factors in the Hh signal pathway, glioma-associated metaplasia to dysplasia to carcinoma in situ and then to invasive oncogene homolog 1 (GLI1), GLI2, and GLI3, function down- carcinoma over 5‑15 years (2). During this time, GBC exhibits stream of SMO, but their biological role in GBC remains few characteristic symptoms, and numerous cases have already unclear. In the present study, the biological significance of developed into locally advanced or metastasized cancer by the GLI1, GLI2, and GLI3 were analyzed with the aim of devel- time of diagnosis. Gemcitabine (GEM), cisplatin (CDDP), and oping novel treatments for GBC.