Effects of Interactions Between the Green and Brown Food Webs on Ecosystem Functioning Kejun Zou
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trophic Levels
Trophic Levels Douglas Wilkin, Ph.D. Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHORS Douglas Wilkin, Ph.D. To access a customizable version of this book, as well as other Jean Brainard, Ph.D. interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2015 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Food Chains in Woodland Habitats. All Animals Need to Eat Food to Survive
Science Lesson Living Things and their Habitats- Food chains in woodland habitats. Key Learning • A food chain shows the links between different living things and where they get their energy from. • Living things can be classified as producers or consumers according to their place in the food chain. • A predator is an animal that feeds on other animals (its prey). • Animals can be described as carnivores, herbivores or omnivores. All animals need to eat food to survive. • Talk about what you already know about the kind of food different animals eat. • What is the name of an animal that only eats plants? • What is the name of an animal that only eats other animals? • What is the name of an animal that eats both plants and other animals? Watch this clip about birds. What kind of food do they eat? https://www.bbc.co.uk/bitesize/clips/z9nhfg8 Animals can be described as herbivores, carnivores or omnivores. Birds like robins, blue tits and house sparrows have a very varied diet! worms spiders slugs flies mealworms berries Robins, blue tits and house sparrows are omnivores because they eat plants and other animals. Describing a food chain. Watch this clip describing a food chain. https://www.bbc.co.uk/bitesize/clips/zjshfg8 Caterpillar cat magpie Think about these questions as you watch • Where does a food chain start? • Which animals are herbivores? • Which animals are carnivores? A food chain starts with energy from the Sun because plants need the Sun’s light energy to make their own food in their leaves. Plants are eaten by animals. -

Ecology (Pyramids, Biomagnification, & Succession
ENERGY PYRAMIDS & Freshmen Biology FOOD CHAINS/FOOD WEBS May 4 – May 8 Lecture ENERGY FLOW •Energy → powers life’s processes •Energy = ATP! •Flow of energy determines the system’s ability to sustain life FEEDING RELATIONSHIPS • Energy flows through an ecosystem in one direction • Sun → autotrophs (producers) → heterotrophs (consumers) FOOD CHAIN VS. FOOD WEB FOOD CHAINS • Energy stored by producers → passed through an ecosystem by a food chain • Food chain = series of steps in which organisms transfer energy by eating and being eaten FOOD WEBS •Feeding relationships are more complex than can be shown in a food chain •Food Web = network of complex interactions •Food webs link all the food chains in an ecosystem together ECOLOGICAL PYRAMIDS • Used to show the relationships in Ecosystems • There are different types: • Energy Pyramid • Biomass Pyramid • Pyramid of numbers ENERGY PYRAMID • Only part of the energy that is stored in one trophic level can be passed on to the next level • Much of the energy that is consumed is used for the basic functions of life (breathing, moving, reproducing) • Only 10% is used to produce more biomass (10 % moves on) • This is what can be obtained from the next trophic level • All of the other energy is lost 10% RULE • Only 10% of energy (from organisms) at one trophic level → the next level • EX: only 10% of energy/calories from grasses is available to cows • WHY? • Energy used for bodily processes (growth/development and repair) • Energy given off as heat • Energy used for daily functioning/movement • Only 10% of energy you take in should be going to your actual biomass/weight which another organism could eat BIOMASS PYRAMID • Total amount of living tissue within a given trophic level = biomass • Represents the amount of potential food available for each trophic level in an ecosystem PYRAMID OF NUMBERS •Based on the number of individuals at each trophic level. -

Plants Are Producers! Draw the Different Producers Below
Name: ______________________________ The Unique Producer Every food chain begins with a producer. Plants are producers. They make their own food, which creates energy for them to grow, reproduce and survive. Being able to make their own food makes them unique; they are the only living things on Earth that can make their own source of food energy. Of course, they require sun, water and air to thrive. Given these three essential ingredients, you will have a healthy plant to begin the food chain. All plants are producers! Draw the different producers below. Apple Tree Rose Bushes Watermelon Grasses Plant Blueberry Flower Fern Daisy Bush List the three essential needs that every producer must have in order to live. © 2009 by Heather Motley Name: ______________________________ Producers can make their own food and energy, but consumers are different. Living things that have to hunt, gather and eat their food are called consumers. Consumers have to eat to gain energy or they will die. There are four types of consumers: omnivores, carnivores, herbivores and decomposers. Herbivores are living things that only eat plants to get the food and energy they need. Animals like whales, elephants, cows, pigs, rabbits, and horses are herbivores. Carnivores are living things that only eat meat. Animals like owls, tigers, sharks and cougars are carnivores. You would not catch a plant in these animals’ mouths. Then, we have the omnivores. Omnivores will eat both plants and animals to get energy. Whichever food source is abundant or available is what they will eat. Animals like the brown bear, dogs, turtles, raccoons and even some people are omnivores. -

Detrital Food Chain As a Possible Mechanism to Support the Trophic Structure of the Planktonic Community in the Photic Zone of a Tropical Reservoir
Limnetica, 39(1): 511-524 (2020). DOI: 10.23818/limn.39.33 © Asociación Ibérica de Limnología, Madrid. Spain. ISSN: 0213-8409 Detrital food chain as a possible mechanism to support the trophic structure of the planktonic community in the photic zone of a tropical reservoir Edison Andrés Parra-García1,*, Nicole Rivera-Parra2, Antonio Picazo3 and Antonio Camacho3 1 Grupo de Investigación en Limnología Básica y Experimental y Biología y Taxonomía Marina, Instituto de Biología, Universidad de Antioquia. 050010 Medellín, Colombia. 2 Grupo de Fundamentos y Enseñanza de la Física y los Sistemas Dinámicos, Instituto de Física, Universidad de Antioquia. 050010 Medellín, Colombia. 3 Instituto Cavanilles de Biodiversidad y Biología Evolutiva. Universidad de Valencia. E–46980 Paterna, Valencia. España. * Corresponding author: [email protected] Received: 31/10/18 Accepted: 10/10/19 ABSTRACT Detrital food chain as a possible mechanism to support the trophic structure of the planktonic community in the photic zone of a tropical reservoir In the photic zone of aquatic ecosystems, where different communities coexist showing different strategies to access one or different resources, the biomass spectra can describe the food transfers and their efficiencies. The purpose of this work is to describe the biomass spectrum and the transfer efficiency, from the primary producers to the top predators of the trophic network, in the photic zone of the Riogrande II reservoir. Data used in the model of the biomass spectrum were taken from several studies carried out between 2010 and 2013 in the reservoir. The analysis of the slope of a biomass spectrum, of the transfer efficiencies, and the omnivory indexes, suggest that most primary production in the photic zone of the Riogrande II reservoir is not directly used by primary consumers, and it appears that detritic mass flows are an indirect way of channeling this production towards zooplankton. -

Chemical Energy And
Unit 6: Energy! From Food to Forces Chemical Energy and LESSON 1 LESSON FOOD CHAIN Unit 6: Energy! From Food to Forces Chemical Energy and LESSON 1 LESSON FOOD CHAIN Food chains and webs show the flow of chemical energy through an ecosystem. From the sun to tertiary consumers. students learn about the transfer of chemical energy and how producers and consumers depend on each other. They also learn scientists classifiy living things based on what they eat. Table of Contents 4 Launch! Sun. Chemical energy passing through the food chain starts with the sun. 6 Chemical Collisions A1: Chemical Energy. Hydrogen and helium are the chemical elements in the sun. 12 Productive Primary Producers A2: Producers. Producers use energy from the sun during photosynthesis. 18 Primary Producer Eaters A3: Primary Consumers. Primary consumers get energy by eating producers. 26 Consuming Critters A4: Secondary Consumers. Secondary consumers get energy by eating primary consumers. 34 Web of Life A5: Food Chains and Webs. Food chains and webs show the transfer of chemical energy in an ecosystem. 50 Tropical Trophic Tiers A6: Energy Pyramid. Scientists show energy transfers from the sun to producers to consumers with trophic levels. Launch! (Sun) SUN! where does chemical energy begin in a food chain? Chemical energy passing through the food chain starts with the sun. Unit 6: Chemical Energy and Food Chain Ready? Materials Nothing to prepare. Sticky notes Pencil Set? • Unit 4-Lesson 1-All Activities: Sun • Unit 6-Lesson 1-Activity 1: Chemical Collisions (Chemical Energy) • Unit 6-Lesson 1-Activity 2: Productive Primary Producers (Producers) • Unit 6-Lesson 1-Activity 3: Primary Producer Eaters (Primary Consumers) • Unit 6-Lesson 1-Activity 4: Consuming Critters (Secondary Consumers) • Unit 6-Lesson 1-Activity 5: Web of Life (Food Chains and Webs) • Unit 6-Lesson 1-Activity 6: Tropical Trophic Tiers (Energy Pyramid) Hawaii Standards Go! SC.K.3.1 Develop Know-Wonder-Learn chart with students. -

The Axial Seamount: Life on a Vent
The Axial Seamount: Life on a Vent Timeframe Description 50 minutes This activity asks students to understand, and build a food web to Target Audience describe the interdependent relationships of hydrothermal vent organisms. Hydrothermal vents were only discovered in 1977, and as Grades 5th- 8th more vents are explored we are finding out more about the unique creatures that live there. In Life on a Vent students will learn about Suggested Materials vent organisms, their feeding relationships, and use that information • Hydrothermal vent organism cards to construct a food web. • Poster paper Objectives • Marking pens Students will: • Sticky notes • Make a food web diagram of the hydrothermal vent community • Painter's tape (to show connections) • Show the flow of energy and materials in a vent ecosystem • Learn about organisms that live in extreme environments and use chemosynthesis to produce energy • Make claims and arguments about each organisms place in the food web Essential Questions What do producers and consumers use as energy at hydrothermal vent ecosystems, and how does that energy travel through the trophic levels of the ecosystem? Background Information Hydrothermal Vents, How do They Form? Under sea volcanoes at spreading ridges and convergent plate boundaries produce underwater geysers, known as hydrothermal vents. They form as seawater seeps deep into the ocean's crust. As the seawater seeps deeper into the Earth, it interacts with latent heat from nearby magma chambers, which are possibly fueling a nearby volcano. Once the freezing cold water heats up deep near the Contact: crust, it begins to rise. As the extremely hot seawater rises, it melts SMILE Program the rocks it passes by leaching chemicals and metals from them [email protected] through high heat chemical reactions. -

Overview Directions
R E S O U R C E L I B R A R Y A C T I V I T Y : 1 H R Marine Food Webs Students investigate marine food webs and trophic levels, research one marine organism, and fit their organisms together in a class-created food web showing a balanced marine ecosystem. G R A D E S 9 - 12+ S U B J E C T S Biology, Ecology, Earth Science, Oceanography, Geography, Physical Geography C O N T E N T S 9 Images, 3 PDFs, 6 Links OVERVIEW Students investigate marine food webs and trophic levels, research one marine organism, and fit their organisms together in a class-created food web showing a balanced marine ecosystem. For the complete activity with media resources, visit: http://www.nationalgeographic.org/activity/marine-food-webs/ DIRECTIONS 1. Build background about marine trophic pyramids and food webs. Review with students that food chains show only one path of food and energy through an ecosystem. In most ecosystems, organisms can get food and energy from more than one source, and may have more than one predator. Healthy, well-balanced ecosystems are made up of multiple, interacting food chains, called food webs. Ask volunteers to come to the front of the room and draw a pyramid and a web. Explain that the shapes of a pyramid and a web are two different ways of representing predator-prey relationships and the energy flow in an ecosystem. Food chains are often represented as food pyramids so that the different trophic levels and the amount of energy and biomass they contain can be compared. -

2 Photosynthesis, Food Chains and Cycles
2 Photosynthesis, food chains and cycles Green plants produce their own food by photosynthesis. All other living organisms depend either directly or indirectly on green plants for their food. This food is passed on from one living organism to the next through food chains. Photosynthesis Photosynthesis is the process by which green plants convert carbon dioxide and water into glucose by using energy from sunlight absorbed by chlorophyll in chloroplasts. Oxygen is produced as a by-product. The process can be summarised by the following word equation: energy from sunlight absorbed carbon dioxide + water glucose + oxygen by chlorophyll Photosynthesis occurs in any plant structure that contains chlorophyll, i.e. which is green; however, it mainly occurs in the leaves. Chlorophyll molecules in the chloroplasts of leaf cells absorb the energy from sunlight and use it to convert carbon dioxide, absorbed from the air, and water, absorbed from the soil, into glucose and oxygen. Fate of the products of photosynthesis The plant uses the oxygen and glucose produced during photosynthesis for various different functions. Oxygen The oxygen is used by the leaf cells in respiration. Excess oxygen diffuses out of the leaves into the air. Glucose The glucose can be used in a variety of ways: • It can be used by the leaf cells in respiration to release energy. • It can be converted to starch by the leaf cells and stored. The starch can then be converted back to glucose and used, e.g. during the night. • It can be converted to other useful organic substances by leaf cells, e.g. -
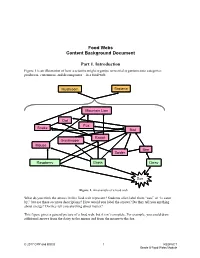
Food Webs Content Background Document Part 1. Introduction
Food Webs Content Background Document Part 1. Introduction Figure 1 is an illustration of how a scientist might organize terrestrial organisms into categories— producers, consumers, and decomposers—in a food web. Mushroom Bacteria Mountain Lion Owl Fox Snake Bird Rabbit Grasshoppe r Mouse Bee Spider Raspberry Grass Daisy Sun Figure 1. An example of a food web What do you think the arrows in this food web represent? Students often label them “eats” or “is eaten by,” but are these accurate descriptions? How would you label the arrows? Do they tell you anything about energy? Do they tell you anything about matter? This figure gives a general picture of a food web, but it isn’t complete. For example, you could draw additional arrows from the daisy to the mouse and from the mouse to the fox. © 2017 CPP and BSCS 1 RESPeCT Grade 5 Food Webs Module STOP AND THINK Why is the Sun present in a food web? Does all the food end up in the mountain lion? Does all the food end up in the bacteria and the mushrooms? Reflect on your answers to the Stop and Think questions as you read the background information and examine how students and scientists answer them. Consider how your understanding changes as you learn more about energy and matter in food webs, and how you will work with your students to align their understandings with those of the science community. 1.1 What Are Food Webs? One way a food web may be described is as a network of food chains that interlink within a biological community. -

Determination of Food Chain Length Using The
WAGENINGEN UNIVERSITY LABORATORY OF ENTOMOLOGY DETERMINATION OF FOOD CHAIN LENGTH USING THE HYPERPARASITOID GELIS AGILIS No: 010.28 Name: Vafia Eirini Study programme: MPS Period: 2 Internship ENT: 80424 1st Supervisor: Rieta Gols 2nd Supervisor: Jeff Harvey Examiner: Marcel Dicke JANUARY 2011 1 ABSTRACT Food chains reflect the interactions among species represented by links, and in turn, the number of links among these species reflects the trophic levels in a food chain. Moreover, the function of species of each trophic level affects other trophic levels above and below them. The interactions among species along with other factors such as the availability of food resources, host-prey size correlations, energy transfer efficiency, community organization, habitat stability and ecosystem size, affect the length of a food chain. Until now researchers have found that food chain length in endotherm-species ecosystems, usually reach no more that four levels. This study determines the food-chain length in an ectothermic-species food-chain, using the hyperparasitoid insect Gelis agilis, chosen for its general feeding preferences. Interestingly, I found out that G. agilis can parasitize and develop on con-specific body tissues, parasitizing host cocoons already parasitized by G. agilis individuals, thus lengthening the food chain up to the 5th and even to the 6th trophic level. The ability of organisms in converting nutrients plays a significant role in their development. This study showed that G. agilis is a highly efficient hyperparasitoid in nutrient conversion, an important factor for food chain length. Moreover, this study also demonstrated the importance of biology and abundance of species in a community, regarding the way these parameters affect food chain length. -

Dynamic Decomposers
Dynamic Decomposers Decomposers aren’t usually the most Grades 3-5 featured part of a food web but they connect the food chain and keep the nutrient and energy cycle flowing. Brought to you by Dynamic Decomposers Grades 3-5 Materials: • Paper • Copies of “trash” cards • Z-Grip Retractable Ballpoint and • Materials for “Decomposition Bottles” Z-Grip Mechanical Pencil in activity description below • Zebra Cadoozles 0.9mm pencil Background: • Decomposers are what is referred to as the FBI (Fungus, Bacteria, and Invertebrates) • They break down plant and animal material and return nutrients to the soil – aka “nature’s recycle team”. • Decomposers aren’t usually the most featured part of a food web but they connect the food chain and keep the nutrient and energy cycle flowing. • For a cool video on decomposition, check out: http://www.pbslearningmedia.org/resource/tdc02.sci.life.oate.decompose/decomposers/ • For a great book about decomposition and composting, check out “Worms Eat My Garbage” by Mary Appelhof. Activities: • Discuss the important role of decomposers and decomposition in a food chain and food web. • Review the importance of the recycling of nutrients back into the ecosystem. • Have your students list decomposers they know of (worms, pillbugs, millipedes, etc) and discuss what they eat. • One cool way we can be conservationists is to keep waste out of landfills. One way we can do this is by composting! • Activity: “Sorting Through the Trash”: • Create a variety of laminated photos of a variety of typical trash items, being sure to include food. Working in pairs or groups, have students sort their photos into 3 categories: 1.