WHO Expert Committee on Drug Dependence Critical Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clearing the Smoke on Cannabis: Regular Use and Cognitive Functioning
6 Clearing the Smoke on Cannabis Regular Use and Cognitive Functioning Robert Gabrys, Ph.D., Research and Policy Analyst, CCSA Amy Porath, Ph.D., Director, Research, CCSA Key Points • Regular use refers to weekly or more frequent cannabis use over a period of months to years. Regular cannabis use is associated with mild cognitive difficulties, which are typically not apparent following about one month of abstinence. Heavy (daily) and long-term cannabis use is related to more This is the first in a series of reports noticeable cognitive impairment. that reviews the effects of cannabis • Cannabis use beginning prior to the age of 16 or 17 is one of the strongest use on various aspects of human predictors of cognitive impairment. However, it is unclear which comes functioning and development. This first — whether cognitive impairment leads to early onset cannabis use or report on the effects of chronic whether beginning cannabis use early in life causes a progressive decline in cannabis use on cognitive functioning cognitive abilities. provides an update of a previous report • Regular cannabis use is associated with altered brain structure and function. with new research findings that validate Once again, it is currently unclear whether chronic cannabis exposure and extend our current understanding directly leads to brain changes or whether differences in brain structure of this issue. Other reports in this precede the onset of chronic cannabis use. series address the link between chronic • Individuals with reduced executive function and maladaptive (risky and cannabis use and mental health, the impulsive) decision making are more likely to develop problematic cannabis use and cannabis use disorder. -

Cannabis Cultivation in the Ancient World (China)
5/23/2019 Cannabis Cultivation in the Ancient World (China) This diagram may be a simplification of the origins of cannabis domestication. Fossil pollen studies may be a better indication of where and when hemp cultivation originated. Cannabis Plant, Hemp Plants and Marijuana plants used Interchangeably. https://friendlyaussiebuds.com/cannabis-resources/education/blazes-throughout-the-ages-episode-i-getting-stoned-in-the-stone-age/ 1 5/23/2019 Approximately 2500 year old cannabis from The Yanghai Tombs in the Turpan Basin (Xinjiang Autonomous Region) of northwest China Approximately 1.7 lbs. of cannabis (A) was found to have characteristic marijuana trichomes (B and C) and a characteristic seed (D). This cannabis contained THC and was presumably employed by this culture as a medicinal or psychoactive agent, or an aid to divination (religious ritual). Ethan Russo et al. Journal of Experimental Botany, Volume 59, Issue 15, 1 November 2008, Pages 4171–4182 Archeological Excavations Yangshao hemp-cordmarked Amphora, Banpo Phase 4800 BCE, Shaanxi. Photographed at the Musee Guimet An amphora is used for storage, usually of liquids 2 5/23/2019 Discovery of Ancient Cannabis cloth Hemp Shoe from China ~ 100CE First hemp-weaved fabric in the World found wrapped around baby in 9,000-year-old house in Turkey The Scythians – the Greeks' name for this initially nomadic people – inhabited Scythia from at least the 11th century BC to the 2nd century AD. In the seventh century BC, the Scythians controlled large swaths of territory throughout Eurasia, from the Black Sea across Siberia to the borders of China. Herodotus[a] (484 BC – 425 BC) was an ancient Greek historian. -

Medical Cannabis Q&A
Medical Cannabis Q&A 1. What is medical cannabis? The term “medical cannabis” is used to describe products derived from the whole cannabis plant or its extracts containing a variety of active cannabinoids and terpenes, which patients take for medical reasons, after interacting with and obtaining authorization from their health care practitioner. 2. What are the main active ingredients? The chemical ingredients of cannabis are called cannabinoids. The two main therapeutic ones are: THC:CBD a. Tetrahydrocannabinol (THC) is a partial agonist of CB1 and CB2 receptors. It is psychoactive and produces the euphoric effect. Each cannabis product will contain THC and CBD, however b. Cannabidiol (CBD) has a weak affinity for CB1 and CB2 receptors and appears the THC: CBD ratio will differ to exert its activity by enhancing the positive effects of the body’s endogenous depending on the product. cannabinoids. 3. Why do patients take it? Medical cannabis may be used to alleviate symptoms for a variety of conditions. It has most commonly been used in neuropathic pain and other chronic pain conditions. There is limited, but developing clinical evidence surrounding its safety and efficacy, and it does not currently have an approved Health Canada indication. 4. How do patients take it? Cannabis can be smoked, vaporized, taken orally, sublingually, topically or rectally. Different routes of administration will result in different pharmacokinetic and pharmacodynamic properties of the drug. 5. Is it possible to develop dependence on medical cannabis? Yes, abrupt discontinuation after long-term use may result in withdrawal symptoms. Additionally, chronic use may result in psychological dependence. -

Cannabis Basics – Glossary of Terms
6/18/2019 Cannabis Basics - Glossary of Terms • Emerald Family Farms EMERALD FAMILY FARMS CANNABIIS BASIICS Cannabis Basics – Glossary of Terms POSTED ON AUGUST 9,, 2018 BY ADMIIN Whether you're a seasoned cannabis connoisseur or just discovering what the world of cannabis has to offer. Battery A rechargeable battery used for heating up a vaporizing cannabis concentrate cartridge. Vape Batteries come in lots of styles and it is important to have one that will heat your cartridge at a proper temperature. Blunt A blunt is a cannabis joint wrapped in tobacco instead of a rolling paper. Created by either dumping out the tobacco of a store-bought cigar then reusing the tobacco wrap for crafting a cannabis joint or crafted using a pre-made blunt wrap. https://www.emeraldfamilyfarms.com/cannabis-basics-glossary/ 1/10 6/18/2019 Cannabis Basics - Glossary of Terms • Emerald Family Farms BHO Butane Hash Oil BHO, butane hash oil is made by pressure blasting cannabis owers with butane. The butane solvent causes THC to become soluble, resulting in a butane/THC mixture. Once the butane is evaporated, depending upon the starting material, apparatus used, and techniques applied during the process, the resulting concentrate product is a viscous and amber colored resin in the form of “sauce,” “crumble,” “wax,” or “shatter.” Bubble Hash A non-solvent based extraction method that utilizes ice-cold temperatures and micron screens to collect trichomes. Called Bubble Hash for its full melt qualities that cause the product to bubble and melt when exposed to ame. Cannabidiol Also known as CBD, is one of at least 113 different cannabinoids found in cannabis. -

Medical Cannabis Cultivation Center Application Illinois Department of Agriculture Springfield, Illinois
Medical Cannabis Cultivation Center Application Illinois Department of Agriculture Springfield, Illinois Schedule 1 – Suitability of the Proposed Facility The following Measures are found in Section 1000.110(b)(1) of the rules: Measure 1: The applicant must demonstrate that the proposed facility is suitable for effective and safe cultivation of medical cannabis, is sufficient in size, power allocation, air exchange and air flow, interior layout, lighting, and sufficient both in the interior and exterior to handle the bulk agricultural production of medical cannabis, cannabis-infused products, product handling, storage, trimming, packaging, loading and shipping. The loading/unloading of medical cannabis in the transport motor vehicle for shipping shall be in an enclosed, secure area out of public sight. Measure 2: The applicant must demonstrate the ability to continue to meet qualifying patient demand by expanding the cultivation facility in a quick and efficient manner with minimal impact on the environment and the surrounding community. Measure 3: The applicant provides an employee handbook that will provide employees with a working guide to the understanding of the day-to-day administration of personnel policies and practices. The following outline is meant as a guide for the applicant to follow in submitting information to meet the above Measures. It is not an all-inclusive list or description of required information. It is the applicant's responsibility to demonstrate compliance with the rules and application instructions. Any engineering drawings, flow diagrams, and descriptions must be adequate to illustrate your plans. 1. Location Area Map (1000.40(e), 1000.100(d)(19), 1000.220(a)) Provide a location map of the area surrounding the facility. -

What Type of Cannabis Therapy Is Best for You?
What to Look For CANNABIS OIL EXTRACTS ü CBD-rich products. Choose products that can be taken orally, sublingually or include both CBD, a non-intoxicating applied topically. Concentrated compound, and THC, the main psychoactive What Type of cannabis oil extracts can also be component of cannabis. CBD and THC work utilized as an ingredient to vaporize or best together, enhancing each other's Cannabis cook with. Some cannabis oils come therapeutic benefits. with an applicator for measured ü dosing. These oil extracts—CBD-rich Clear labels. Look for product labels Therapy Is showing the quantity and ratio of CBD and and THC-dominant—are very potent. THC per dose, a manufacturing date and The time of onset and duration of batch number (for quality control). Best for You? effect vary depending on the method of administration. ü Lab testing. Look for products that are tested for consistency, and verified as free of mold, bacteria, pesticides, solvent residues, and other contaminants. ü Quality ingredients. Select products with Visit ProjectCBD.org for: quality ingredients. (No corn syrup, GMOs, transfats, preservatives, and artificial CBD Locator • Educational resources additives.) • Dispensary staff training • Updates ü Safe extraction. Avoid products extracted on cannabis science & therapeutics • with toxic solvents like BHO, propane, hexane CBD-rich product list • Analysis of or other hydrocarbons. Solvent residues are industry trends • Events • especially dangerous for immune- Announcements • Referrals compromised patients. Look for products that entail a safer method of extraction like supercritical CO2. Advancing whole plant ü Products made from organic cannabis not industrial hemp. Compared to high resin cannabis therapeutics cannabis, hemp is typically low in cannabinoid content. -

Extracts and Tinctures of Cannabis
WHO Expert Committee on Drug Dependence Critical Review …………….. Extracts and tinctures of cannabis This report contains the views of an international group of experts, and does not necessarily represent the decisions or the stated policy of the World Health Organization © World Health Organization 2018 All rights reserved. This is an advance copy distributed to the participants of the 41st Expert Committee on Drug Dependence, before it has been formally published by the World Health Organization. The document may not be reviewed, abstracted, quoted, reproduced, transmitted, distributed, translated or adapted, in part or in whole, in any form or by any means without the permission of the World Health Organization. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use. -

Methods to Assess Cannabis Consumption in Population Surveys
QHRXXX10.1177/1049732318820523Qualitative Health ResearchGoodman et al. 820523research-article2019 Research Article Qualitative Health Research 1 –9 Methods to Assess Cannabis © The Author(s) 2019 Article reuse guidelines: sagepub.com/journals-permissions Consumption in Population Surveys: DOI:https://doi.org/10.1177/1049732318820523 10.1177/1049732318820523 Results of Cognitive Interviewing journals.sagepub.com/home/qhr Samantha Goodman1 , Cesar Leos-Toro1, and David Hammond1 Abstract The Cannabis Act legalized the possession and sale of nonmedical cannabis in Canada on October 17, 2018. Evaluating the impact of cannabis legalization requires a more thorough understanding than is provided by most existing measures of cannabis use. The aim of this study was to pretest a range of cannabis consumption measures used in a population- based survey and to share insights gained in the process. Cognitive interviewing was conducted among 10 cannabis users aged ≥16 years. Comprehension and self-reporting of consumption types and amounts, sources of purchase, and cannabinoid levels were examined. Findings revealed areas for improvement in a number of survey items, including unclear wording and reference images. Identified issues were used to improve the survey for use in the International Cannabis Policy Study. The authors discuss important principles (e.g., use of visual cues, user-selected units, and time frames) that should be adopted when assessing cannabis use in population-based studies. Keywords cannabis; cognitive interview; prevalence; measurement; qualitative; Canada Background cannabis consumption in terms of the number of times cannabis is used within a particular month, day or week, Cannabis (also referred to as “marijuana,” “pot,” “weed,” or the typical amount used on each occasion (e.g., etc.) is the most frequently used illicit substance world- Canadian Cannabis Survey; Advanis, 2017). -

Williamstown Cannabis Cultivation Business Plan
Williamstown Cannabis Cultivation Business Plan Davis Collison and Rosa Kirk-Davidoff We are on the stolen land of the Stockbridge-Munsee Band of the Mohican. “The legal marijuana industry has the potential to save local farms and repair a broken food system.”- Suehiko Ono, EOS Farms Introduction Averill Cook Davis and Rosa ● Who we are ● Environmental Planning ○ Senior Seminar for Environmental Studies Majors ● This project - Williamstown Cannabis Cultivation ● Questions: Best scale to start? Opportunities for a craft market? Jake Zieminski Our Clients ● Averill H Cook ○ Born and raised in Williamstown. ○ BS degree from University of Vermont ○ Owned and operated a pellet manufacturing business for 12 years ○ Traveled throughout numerous countries consulting in wood energy ○ Maintained an operated Wendling Farm in Williamstown where he grew up ○ Superior land stewardship has been paramount throughout his career Averill Cook Jake Zieminski Our Clients ● Jake Zieminski ○ Born and raised in Cheshire Ma on family dairy farm. Lived in Boston for 20 years and recently moved family back in 2018 to launch cannabis start-up. ○ Cannabis Entrepreneur ■ Current owner of CAVU Hemp, Cheshire Ma- MDAR licensed 2019 ■ 2021- CCC – Marijuana Cultivation Applicant ■ Cannabis Activist, Educator and Advisor since 2014 ○ Prior to transitioning into Cannabis industry in 2018, Mr. Zieminski was a management consultant focused in healthcare. Mr. Zieminski has spent the primary part of his career in client based performance improvement roles at PricewaterhouseCoopers(PwC), -

Cannabinoid As Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity
Cannabinoid as Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity Sudipta Baroi1, Achintya Saha2, Ritesh Bachar3 and Sitesh C Bachar4 1Department of Pharmaceutical Technology, Faculty of Pharmacy, University of Dhaka Dhaka-1000, Bangladesh 2Department of Chemical Technology, Pharmaceutical & Fine Chemical Technology Division University of Calcutta, India 3Department of Pharmacy, School of Science and Engineering, University of Information Technology and Sciences (UITS), Dhaka-1212, Bangladesh 4Department of Pharmacy, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh (Received: April 15, 2020; Accepted: June 2, 2020; Published (web): June 28, 2020) ABSTRACT: Inhibition of aromatase (CYTP450), a key enzyme in the estrogen biosynthesis, could result in regression of estrogen-dependent tumors and even prevent the promotion of breast cancer. The present research has been designed for searching a potent chemical moiety from natural sources to inhibit aromatase enzyme, the over- functionality of which causes the breast cancer. Cannabis sativa contains a very much promising group of cannabinoids with more than 66 compounds with reported anticancer property and for the search of a target specific potent aromatase inhibitor, 61 cannabinoids from C. sativa were selected. The Structures Data File (SDF) of these ligand molecules were subjected to docking studies at the binding site of aromatase X-ray crystallographic structure based on lower resolution of the protein crystal structure and higher docking accuracy, predicted by calculating the correlation between experimental activities and Glide dock scores and compared with the standard aromatase ligand androstenedione and aromatase inhibitor fadrozole with existing drug for breast cancer treatment. The best docked pose of each ligand was selected on the basis of the highest dock score related to the binding free energies of the internal dataset compounds as compared to their observed activities. -

Personal Use Cannabis Rules Special Adopted New Rules: N.J.A.C
NEW JERSEY CANNABIS REGULATORY COMMISSION Personal Use Cannabis Rules Special Adopted New Rules: N.J.A.C. 17:30 Adopted: August 19, 2021 by New Jersey Cannabis Regulatory Commission, Dianna Houenou, Chair. Filed: August 19, 2021 Authority: N.J.S.A. 24:6I-31 et seq. Effective Date: August 19, 2021 Expiration Date: August 19, 2022 This rule may be viewed or downloaded from the Commission’s website at nj.gov/cannabis. These rules are adopted pursuant to N.J.S.A. 24:6I-34(d)1a of the New Jersey Cannabis Regulatory, Enforcement Assistance, and Marketplace Modernization Act, N.J.S.A. 24:6I- 31 et seq., and became effective upon acceptance for filing by the Office of Administrative Law. The specially adopted new rules shall be effective for a period not to exceed one year from the date of filing of the new rules, that is, until August 19, 2022. The Commission has provided this special adoption to the Attorney General, State Treasurer, Commissioner of Health, and Commissioner of Banking and Insurance for a consultation period, after which the Commission anticipates filing a proposal to readopt these rules with amendments reflecting the results of that consultation. In accordance with N.J.S.A. 24:6I-34(d)1b the rules, as readopted, will become effective upon acceptance for filing by the Office of Administrative Law if filed on or before the expiration date of the rules published herein. The adopted amendments will be effective upon publication in the New Jersey Register. Federal Standards Analysis The Cannabis Regulatory, Enforcement Assistance, and Marketplace Modernization Act obliges the Cannabis Regulatory Commission to promulgate rules necessary or proper to enable it to carry out the Commission’s duties, functions, and powers with respect to overseeing the development, regulation, and enforcement of activities associated with the personal use of cannabis pursuant to P.L.2021, c.16. -
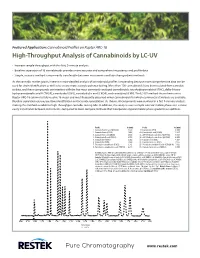
High-Throughput Analysis of Cannabinoids by LC-UV
Featured Application: Cannabinoid Profiles on Raptor ARC-18 High-Throughput Analysis of Cannabinoids by LC-UV • Increase sample throughput with this fast, 9-minute analysis. • Baseline separation of 16 cannabinoids provides more accurate and comprehensive potency and profile data. • Simple, isocratic method is more easily transferable between instruments and labs than gradient methods. As the cannabis market grows, interest in more detailed analysis of cannabinoid profiles is expanding because more comprehensive data can be used for strain identification as well as to ensure more accurate potency testing. More than 100 cannabinoids have been isolated from cannabis to date, and these compounds can interfere with the five most commonly analyzed cannabinoids: tetrahydrocannabinol (THC), delta-9-tetra- hydrocannabinolic acid A (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), and cannabinol (CBN). The LC-UV method shown here uses a Raptor ARC-18 column to fully resolve 16 major and most frequently observed minor cannabinoids for which commercial standards are available. Baseline separation ensures positive identification and accurate quantitation. As shown, all compounds were resolved in a fast 9-minute analysis, making this method suitable for high-throughput cannabis testing labs. In addition, this analysis uses a simple isocratic mobile phase so it is more easily transferable between instruments, compared to more complex methods that incorporate atypical mobile phase gradients or additives. Peaks tR (min) Peaks tR (min) 1. Cannabidivarinic acid (CBDVA) 1.877 9. Cannabinol (CBN) 4.609 2. Cannabidivarin (CBDV) 2.086 10. Cannabinolic acid (CBNA) 5.437 3. Cannabidiolic acid (CBDA) 2.592 11. Δ9-Tetrahydrocannabinol (Δ9-THC) 5.815 4. Cannabigerolic acid (CBGA) 2.750 12.