2011 Progress Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inspections and Inspectors 4 NEW.Xlsx
Inspector listing 20/09/2019 13:36 1/12 Name Surname Hospital City Country Inspector area(s) Paedi Status Languag atrics es ? Gerhard Fritsch St. Anna Kinderspittal Vienna Austria Processing Active DE;EN Peter Kalhs Medizinische Universitaet Wien Vienna Austria Clinical Active DE;EN Peter Schlenke Medizinische Universität Graz Graz Austria Collection/Processing Active DE;EN Volker Witt St. Anna Kinderspital Vienna Austria Clinical/Collection/Processing Active DE;EN Nina Worel Medizinische Universitaet Wien Vienna Austria Collection/Processing Active DE;EN Cindy Aerts UZ Brussel Brussels Belgium Quality Management Active NL Maria Bordon Cueto Ghent University Hospital Gent Belgium Clinical yes Active NL;EN;E Dries Deeren Roeslaere Belgium Clinical/Collection Active EN;NL Evelyne Dewulf AZ Delta Roeslaere Belgium Quality Management Active NL;EN Alain Gadisseur Edegem Edegem Belgium Clinical Active NL; EN Phuong Huynh Institut Jules Bordet Brussels Belgium Collection/Quality Active FR;VT Erwin Janssen ZNA Antwerp Antwerp Belgium Quality Management Active NL;EN Jessy Lardon ZNA Stuivenberg Antwerp Belgium Processing Active EN;NL Dominiqu Latinne Service of Bioclinical hematology St.Luc Brussels Belgium Processing Active EN;NL;F Alexandrin Maes Institution UZ Gent Gent Belgium Quality Management Active EN;NL;F Marleen Renard Univerity Hospital Leuven Leuven Belgium Clinical Yes Active EN;NL;F Olivier Urbain Cliniques Universitaires Saint Luc Brussels Belgium Quality Management Active FR; EN Ivan Van Riet Academic Hospital Free University Brussels -

Scientific Programme Saturday, 17 March 2018 Patient, Family and Donor Day, Session 1 Coffee Break Patient, Family and Donor
44th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), 17 - 21 March 2018, Lisbon, Portugal Scientific Programme Saturday, 17 March 2018 Patient, Family Donor Day 09:25 - 10:30 Room 3A + Room 3B Patient, Family and Donor Day, Session 1 Chairs: Manuel Abecasis (Portugal) Welcome and Introduction 09:25 - 09:30 Manuel Abecasis (Portugal) What is a hematopoietic transplant? 09:30 - 09:50 Carlos Martins (Portugal) Choice of the stem cell product for transplant 09:50 - 10:10 Maria João Gutierrez (Portugal) Different progression phases of the transplant process 10:10 - 10:30 Filipa Moita (Portugal) 10:30 - 11:00 Room 3A + Room 3B Coffee Break Patient, Family Donor Day 11:00 - 12:30 Room 3A + Room 3B Patient, Family and Donor Day, Session 2 Chairs: Fernanda Soares (Portugal) Nursing care of the patient in the isolation unit 11:00 - 11:20 Elsa Oliveira (Portugal) Nursing care of the patient in the day unit 11:20 - 11:40 Cristina Santos (Portugal) The patient through the transplant process 11:40 - 12:10 Claudia Franco (Portugal) Eva Fraio (Portugal) Carlos Horta E Costa (Portugal) Cultural Moment - Guitar 12:10 - 12:30 João Lopes (Portugal) Francisco Palma (Portugal) Page 1 / 202 44th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), 17 - 21 March 2018, Lisbon, Portugal Scientific Programme 12:30 - 13:30 Room 3A + Room 3B Lunch Break Patient, Family Donor Day 13:30 - 14:45 Room 3A + Room 3B Patient, Family and Donor Day, Session 3 Chairs: Helder Trindade (Portugal) Menachem Bitan -

Regular Article
From www.bloodjournal.org by guest on September 11, 2016. For personal use only. Regular Article LYMPHOID NEOPLASIA Autologous/reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: long-term results of the EBMT-NMAM2000 study G¨osta Gahrton,1 Simona Iacobelli,2 Bo Bj¨orkstrand,1 Ute Hegenbart,3 Astrid Gruber,1 Hildegard Greinix,4 Liisa Volin,5 Franco Narni,6 Angelo Michele Carella,7 Meral Beksac,8 Alberto Bosi,9 Giuseppe Milone,10 Paolo Corradini,11 Stefan Sch¨onland,3 Kristina Friberg,1 Anja van Biezen,12 Hartmut Goldschmidt,3 Theo de Witte,13 Curly Morris,14 Dietger Niederwieser,15 Laurent Garderet,16 and Nicolaus Kr¨oger,17 for the EBMT Chronic Malignancies Working Party Plasma Cell Disorders Subcommittee 1Department of Medicine, Karolinska Institute at Huddinge and Solna, Stockholm, Sweden; 2Centro Interdipartimentale di Biostatistica e Bioinformatica, Universit`a di Roma Tor Vergata, Italy; 3University of Heidelberg, Germany; 4Medical University of Vienna, Austria; 5Helsinki University Central Hospital, Finland; 6University of Modena, Italy; 7CSS Hospital IRCCS, San Giovanni Rotondo, Italy; 8Ankara University Faculty of Medicine, Turkey; 9Ospedale di Careggi, Florence, Italy; 10Ospedale Ferrarotto, Catania, Italy; 11University of Milano, Italy; 12Leiden University Medical Center, The Netherlands; 13Radboud University Nijmegen Medical Center, The Netherlands; 14The Queens University, Belfast, United Kingdom; 15University Hospital Leipzig, Germany; 16Hopital St Antoine, Paris, France; -

Inizia Un Esilio Che Non Si Sa Quando Avrà Fine
^x^ Quotidiano / Anno LUI / N. 204 (%? fó'ì ) * Martedì 27 luglio 1976 / 1,150 . ,££& Attuato lo sgombero della zona avvelenata v LE PRIME FAMIGLIE ORGANO DEL PARTITO COMUNISTA ITALIANO LASCIANO SEVESO Entro 48 ore decisione di Andreotti sul mandato ricevuto Governo centrale e governo locale Fase cruciale della crisi Inizia un esilio EL momento in cui com salali anziché lo sfrenato N pilava i suoi appunti consumismo di beni indivi che non si sa per un programma di go duali voluto dalla logica del verno l'on. Andreotti non profitto e della specula aveva ancora incontrato le zione. delegazioni dei sindaci, dei presidenti di provincia e di E non paia strano che ^^Mne. Immagino che quan con tanta forza si ponga u- Oggi la Direzione della DC quando avrà fine do dovrà dare a quei suoi na questione di adeguamen appunti sistemazione defini to e di rinnovamento isti tiva per sottoporli al giudi tuzionale, la questione dei Consultazioni di Zaccagnini con i dirigenti de: voci su alcuni contrasti e di La gente è disciplinala ma non face il suo sdegno - «Adesso, dopo due set zio ed al voto delle Came poteri appunto, in un mo re, il presidente designato mento di così grave crisi spareri, e indiscrezioni sulla ipotesi della soluzione monocolore - La posizio timane, ci fanno scappare senza neanche ii tempo di fare una telefonata» a formare il governo nazio economica, politica e socia Per tanti è anche un dramma economico - Si abbattono gli animali più maiali nale non potrà non ripensa le, perché la riforma dello ne dei socialisti, dei socialdemocratici e del PRI - Un'intervista di Macaluso re a quanto gli hanno det ordinamento dello Stato è to e gli diranno i rappre condizione indispensabile, è Che la crisi di governo sia giunta a un punto cruciale, è sottolineato dall'incalzare dello stesso calendario politico. -

La Vela a Tokyo 2020
Carissimi, siamo finalmente giunti a questo appuntamento Olimpico! Lo Sport – nella più nobile delle sue funzioni – potrà riunire tutti sotto un’unica bandiera: quella della solidarietà, del rispetto e della sana competizione. Questi lunghi mesi che ci stiamo lasciando faticosamente alle spalle hanno severamente provato il nostro spirito e ci hanno costretto a considerare in maniera più genuina i rapporti umani, le priorità della nostra vita, i nostri affetti. Lo Sport in questo contesto ha rafforzato la sua valenza sociale dimostrandosi un collante e nel contempo una valvola di sfogo. Questi Giochi devono e vogliono rappresentare una rinascita verso un graduale ritorno alla normalità, senza però dimenticare quanto accaduto. Preparare una spedizione Olimpica non è affatto semplice, soprattutto in uno sport come il nostro dove la preparazione atletica è solo uno dei molteplici componenti: meteorologia, tecnologia, programmazione, logistica, gestione delle risorse sono solo alcuni degli aspetti che attraversano in maniera trasversale il mondo della vela. Per questo vorrei ringraziare tutti coloro che hanno partecipato alla formazione di questa squadra: il Direttore Tecnico Michele Marchesini, tutti i Tecnici che in questo quadriennio hanno lavorato meticolosamente, il Team Manager Guglielmo Vatteroni, e tutto il comparto tecnico, medico e atletico dei collaboratori. Per ultimo – ma non certo per importanza, visto che questa pubblicazione è dedicata proprio a loro – il mio pensiero va ai ragazzi che ci rappresenteranno ai Giochi Olimpici: questi anni di preparazione li hanno visti maturare come atleti ma soprattutto sotto il profilo umano. Il mio augurio è che possano raccogliere il massimo da questa esperienza che rimane uno dei momenti più alti e indimenticabili nella vita di uno sportivo. -
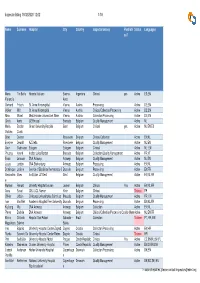
Inspector Listing 18/02/2020 12:02 1/16
Inspector listing 18/02/2020 12:02 1/16 Name Surname Hospital City Country Inspector area(s) Paediatri Status Languages cs? Maria Tisi Baña Hospital Italiano Buenos Argentina Clinical yes Active ES;EN Florencia Aires Gerhard Fritsch St. Anna Kinderspittal Vienna Austria Processing Active DE;EN Volker Witt St. Anna Kinderspital Vienna Austria Clinical/Collection/Processing Active DE;EN Nina Worel Medizinische Universitaet Wien Vienna Austria Collection/Processing Active DE;EN Cindy Aerts UZ Brussel Brussels Belgium Quality Management Active NL Maria Bordon Ghent University Hospital Gent Belgium Clinical yes Active NL;EN;ES Victoria Cueto Dries Deeren Roeslaere Belgium Clinical/Collection Active EN;NL Evelyne Dewulf AZ Delta Roeslaere Belgium Quality Management Active NL;EN Alain Gadisseur Edegem Edegem Belgium Clinical Active NL; EN Phuong Huynh Institut Jules Bordet Brussels Belgium Collection/Quality Management Active FR;VT Erwin Janssen ZNA Antwerp Antwerp Belgium Quality Management Active NL;EN Jessy Lardon ZNA Stuivenberg Antwerp Belgium Processing Active EN;NL Dominique Latinne Service of Bioclinical hematology St.LucBrussels Belgium Processing Active EN;FR Alexandrin Maes Institution UZ Gent Gent Belgium Quality Management Active EN;NL;FR e Marleen Renard Univerity Hospital Leuven Leuven Belgium Clinical Yes Active EN;NL;FR Anne Sonet CHU UCL Namur Yvoir Belgium Clinical Trainee FR Olivier Urbain Cliniques Universitaires Saint Luc Brussels Belgium Quality Management Active FR; EN Ivan Van Riet Academic Hospital Free University BrusselsBrussels -

Novodobé Olympijské
SLOVENSKÝ ZVÄZ JACHTINGU OLYMPIJSKÉ HRY 1896 - 2004 JACHTING BRATISLAVA 2004 2 Ú V O D V roku 2004 sa XXVIII. Olympijské hry vrátili späť do svojej kolísky, do Grécka. Samo- zrejme nie na pôvodné miesto do Olympie, kde sa konali v staroveku po viac ako tisíc rokov, ale do hlavného mesta slobodného Grécka do novodobých Atén. Splnila sa dávna túžba Grékov a ich hlavného mesta, aby po prvých novodobých hrách, ktoré sa uskutočnili v roku 1896 sa ďalšie ko- nali v krajine ich zrodu. Je treba privítať rozhodnutie Medzinárodného olympijského výboru o pridelení XXVIII. hier do Atén, aj keď uskutočnenie takého gigantického športového podniku akým sú OH, vytvorilo viaceré ťažkosti pri ich realizácii tak pre organizátorov, ako aj pre vládu Grécka. Vzdor všetkým ťažkostiam sa Grékom podarilo zvládnuť všetky problémy a pripravili pre športovcov celého sveta prostredie, aké bolo hodné nasledovníkov športovcov starovekej He- lady. Jachting je jedným z málo športov, ktorý bol vypísaný ako športová disciplína okrem III. OH v Saint Luis na všetkých doterajších OH. V programe hier už pre prvé novodobé hry v roku 1896 v Aténach bol jachting vypísaný v rámci vodných športov, no olympijská regata sa nekonala pre zlé počasie. Nás jachtárov samozrejme zaujímajú najviac jachtárske olympijské regaty, v nich vypísané lodné triedy a typy plachetníc na ktorých sa olympijské súťaže uskutočňovali. Je to tiež príležitosť, aby sme nazreli do doterajších výsledkových listín Olympijských hier a dozvedeli sa, kto boli ich víťazi. V roku 1982 vo zväzku 26. "SLOVENSKÉHO JACHTÁRA", ktorý bol celý venovaný jach- társkemu olympizmu z príležitosti uskutočnenej olympijskej regaty v Talline (OH Moskva 1980), bol Otom Malým spracovaný neúplný prehľad všetkých dovtedajších regát na OH, vrátane tried v ktorých sa pretekalo a ich víťazov. -

2019 LOG STAR CLASS CLASS STAR ASSOCIATION YACHT RACING RACING YACHT INTERNATIONAL INTERNATIONAL Honoring the Past, Leading the Future Leading the Past, Honoring
INTERNATIONAL STAR CLASS 2019 LOG YACHT RACING ASSOCIATION Honoring the Past, Leading the Future 2019 LOG Star Class Merchandise available online or from the Central Offi ce www.starclass.org/fl eet-tools - Merchandise Shop Ties & Scarves Star class ties and scarves: a great tradition to give to District & Silver Star winners at your regattas! WITH HELPWITH FROM HELP THEFROM QUANTUM THE QUANTUM TEAM TEAM Gold Star - World champions Silver Star - Silver Star champions Blue Star - District champions Green Star - Green champions Red Star - available for all Ties: $40 Scarves (limited qty): $50 Star Class Blazer Patches Window decal & Historical (left): $30 patch Classic (middle): $30 Jacket Patch: $5 Window decal: $5 Belts & Key Fob Contact your local loft or one of our Star class experts: Contact your local loft or one of our Star class experts: Belts -d-ring or Mark Reynolds | [email protected] buckle: $30 Mark Reynolds | [email protected] George Szabo | [email protected] George Szabo | [email protected] Key fob: $5 Alexandre Paradeda | [email protected] Alexandre Paradeda | [email protected] Vittorio d’Albertas | [email protected] Vittorio d’Albertas | [email protected] ISCYRA fl ag small: $35 (12 x 18”-31cm x 46cm) medium: $50 (24 x 36” -62cm x 92cm) large: $80 (48 x 72” -123cm x 184cm) Gold Stars: 2017, 2016, 2014, 2009, 2008, 2007, 2006, 2005, 2004, 2003, 2001, 2000, 1998, Lapel Pin-$5 1997, 1996, 1995, 1993, 1992, 1991, 1987 Gold Stars: 2017, 2016, 2014, 2009, 2008, 2007, 2006, 2005, 2004, 2003, 2001, 2000, 1998, Class History - $15 First Place: 20171997, Star 1996, Worlds 1995, (partial),1993, 1992, 2018 1991, Bacardi 1987 Cup, 2018 Western Hemispheres, 2018 Eastern Hemispheres First Place: 2017 Star Worlds (partial), 2018 Bacardi Cup, 2018Photo Western by Andrea Hemispheres, Falcon. -

Annual Report and Financial Statements Financial Year 2016
Annual report and Financial Statements Financial Year 2016 SHAREHOLDERS’ MEETING ON APRIL 22ND, 2017 121st FINANCIAL YEAR Please note that the original Report is in Italian. In case of doubt the Italian version prevails. Contents Group Structure 9 Calling of the ordinary and extraordinary shareholders’ meeting 15 Corporate Bodies 21 Reference Scenario 23 Management Report 41 The Company in 2016 43 Highlights 45 2014-2017 Business Plan 53 Ways in which the Group image and information are disclosed 58 Significant events during the year 59 Insurance business 67 Premiums by sector of acquisition 69 Non-life business 70 Claims settlement 77 Life business 79 Claims paid 82 Research and development activities - new products 83 Reinsurance 85 Financial and asset management 91 Investment property 93 Securities investments 94 Solvency II ratio 97 Analysis of the financial risks 97 Headcount and sales network 101 Human resources 103 Sales network 106 Other information 111 Corporate governance and internal control system 113 Prevention and countering fraud 113 Complaints management 114 Disclosure on Solvency II fulfilments 114 Information systems 115 Appointments of senior management of the Company 116 Significant events during the first few months of 2017 117 3 Atypical or unusual transactions and non-recurrent significant operations and events 118 Transactions with related parties 118 Management and co-ordination activities according to Article 2497 et seq. of the Italian Civil Code 118 Tax consolidation 119 Shareholders 119 Own shares 120 Newly