University of Florida Thesis Or Dissertation Formatting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
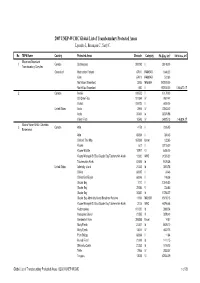
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C. No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Ellesmere/Greenland 1 Canada Quttinirpaaq 300093 II 38148.00 Transboundary Complex Greenland Hochstetter Forland 67910 RAMSAR 1848.20 Kilen 67911 RAMSAR 512.80 North-East Greenland 2065 MAB-BR 972000.00 North-East Greenland 650 II 972000.00 1,008,470.17 2 Canada Ivvavik 100672 II 10170.00 Old Crow Flats 101594 IV 7697.47 Vuntut 100673 II 4400.00 United States Arctic 2904 IV 72843.42 Arctic 35361 Ia 32374.98 Yukon Flats 10543 IV 34925.13 146,824.27 Alaska-Yukon-British Columbia 3 Canada Atlin 4178 II 2326.95 Borderlands Atlin 65094 II 384.45 Chilkoot Trail Nhp 167269 Unset 122.65 Kluane 612 II 22015.00 Kluane Wildlife 18707 VI 6450.00 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 12200 WHC 31595.00 Tatshenshini-Alsek 67406 Ib 9470.26 United States Admiralty Island 21243 Ib 3803.76 Chilkat 68395 II 24.46 Chilkat Bald Eagle 68396 II 198.38 Glacier Bay 1010 II 13045.50 Glacier Bay 22485 V 233.85 Glacier Bay 35382 Ib 10784.27 Glacier Bay-Admiralty Island Biosphere Reserve 11591 MAB-BR 15150.15 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 2018 WHC 66796.48 Kootznoowoo 101220 Ib 3868.24 Malaspina Glacier 21555 III 3878.40 Mendenhall River 306286 Unset 14.57 Misty Fiords 21247 Ib 8675.10 Misty Fjords 13041 IV 4622.75 Point Bridge 68394 II 11.64 Russell Fiord 21249 Ib 1411.15 Stikine-LeConte 21252 Ib 1816.75 Tetlin 2956 IV 2833.07 Tongass 13038 VI 67404.09 Global List of Transboundary Protected Areas ©2007 UNEP-WCMC 1 of 78 No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Tracy Arm-Fords Terror 21254 Ib 2643.43 Wrangell-St Elias 1005 II 33820.14 Wrangell-St Elias 35387 Ib 36740.24 Wrangell-St. -

Lake Tanganyika, Regional Fisheries Programme (TREFIP)
FAO/NORWAY GOVERNMENT GCP/INT/648/NOR COOPERATIVE PROGRAMME Field Report F-14 (En) eries FISHCODE MANAGEMENT LAKE TANGANYIKA REGIONAL FISHERIES PROGRAMME (TREFIP) PREPARED BY THE JOINT AfDB/FAO/FISHCODE MISSION C. MAGNET, J.E. REYNOLDS AND H. BRU FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS ROME, JULY 2000 FAO/Norway Programme of Assistance to Developing Countries for the Implementation of the Code of Conduct for Responsible of the Code Conduct FAO/NorwayFish Programme of Assistance to Developing Countries for the Implementation Fisheries Management for the Provision Advice of Scientific for Improving Countries to Developing Assistance F: Sub-programme LAKE TANGANYIKA REGIONAL FISHERIES PROGRAMME (TREFIP) A proposal for implementation of the Lake Tanganyika Framework Fisheries Management Plan Prepared by: The Joint AfDB/FAO/FISHCODE Lake Tanganyika Mission Christophe Magnet (Team Leader/Economist, AfDB), J.Eric Reynolds (Development Planner/Socio-Economist, FAO), & Hervé Bru (Infrastructure/Marketing Specialist, AfDB) African Development Bank, Food and Agriculture Organization Abidjan of the United Nations, Rome July 2000 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. LAKE TANGANYIKA REGIONAL FISHERIES PROGRAMME (TREFIP) 18.07.00 ACKNOWLEDGEMENTS This document was drafted on behalf of the AfDB and the four Lake Tanganyika littoral States of Burundi, the Democratic Republic of Congo (DRC), Tanzania, and Zambia. Responsibility for its preparation was assigned to the Fisheries Policy and Planning Service (FIPP) of FAO, with funding provided by the AfDB and the FAO FISHCODE Programme (GCP/INT/648/NOR -- Interregional Programme of Assistance to Developing Countries for the Implementation of the Code of Conduct for Responsible Fisheries). -

Ecological Changes in the Zambezi River Basin This Book Is a Product of the CODESRIA Comparative Research Network
Ecological Changes in the Zambezi River Basin This book is a product of the CODESRIA Comparative Research Network. Ecological Changes in the Zambezi River Basin Edited by Mzime Ndebele-Murisa Ismael Aaron Kimirei Chipo Plaxedes Mubaya Taurai Bere Council for the Development of Social Science Research in Africa DAKAR © CODESRIA 2020 Council for the Development of Social Science Research in Africa Avenue Cheikh Anta Diop, Angle Canal IV BP 3304 Dakar, 18524, Senegal Website: www.codesria.org ISBN: 978-2-86978-713-1 All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording or any information storage or retrieval system without prior permission from CODESRIA. Typesetting: CODESRIA Graphics and Cover Design: Masumbuko Semba Distributed in Africa by CODESRIA Distributed elsewhere by African Books Collective, Oxford, UK Website: www.africanbookscollective.com The Council for the Development of Social Science Research in Africa (CODESRIA) is an independent organisation whose principal objectives are to facilitate research, promote research-based publishing and create multiple forums for critical thinking and exchange of views among African researchers. All these are aimed at reducing the fragmentation of research in the continent through the creation of thematic research networks that cut across linguistic and regional boundaries. CODESRIA publishes Africa Development, the longest standing Africa based social science journal; Afrika Zamani, a journal of history; the African Sociological Review; Africa Review of Books and the Journal of Higher Education in Africa. The Council also co- publishes Identity, Culture and Politics: An Afro-Asian Dialogue; and the Afro-Arab Selections for Social Sciences. -

Pangani Basin: a Situation Analysis
Pangani Basin: A Situation Analysis IUCN Eastern Africa Programme 2003 i Published by: Copyright: © 2003 International Union for Conservation of Nature and Natural Resources This publication may be produced in whole or part and in any form for education or non-profit uses, without special permission from the copyright holder, provided acknowledgement of the source is made. IUCN would appreciate receiving a copy of any publication which uses this publication as a source. No use of this publication may be made for resale or other commercial purpose without the prior written permission of IUCN. Citation: IUCN Eastern Africa Programme, 2003. The Pangani River Basin: A Situation Analysis, xvi + 104pp. ISBN: 2-8317-0760-9 Design and layout: Gordon O. Arara Printed by: ScanHouse Press Ltd. Photo 1: The summit of Mount Kilimanjaro; Photo 2: Forest stand at 1 Shire Njoro; Photo 3: Gate controlling the release of water into irrigation furrows; Photo 4: Children swimming in an irrigation 3 4 reservoir; Photo 5: Sisal plantations; Photo 6: Irrigated rice scheme; 2 Photo 7: Water gauging station at Chemka Spring; Photo 8: Vandalized gate controlling the release of water into irrigation furrows; Photo 9: 5 Dam wall at Nyumba ya Mungu Reservoir (color changes mark the declining water levels); Photo 10: A vendor sells water from a borehole 6 9 10 Photos 1, 3, 5, 6, 8, 9 copyright 2003 Kelly West; Photos 2, 7 7 8 copyright 2002 Kim Geheb; Photos 4, 10 copyright 2003 Ger Bergkamp. Available from: IUCN- EARO Publications Service Unit, P. O. Box 68200 - 00200, Nairobi, Kenya; Telephone ++ 254 20 890605-12; Fax ++ 254 20 890615; E-mail: [email protected] The designations of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of the participating organiza- tions concerning the legal status of any country, territory, or area, or of its authorities, or con- cerning the delimitation of its frontiers or boundaries. -

Technical Report: Second Order Water Scarcity in Southern Africa
Second Order Water Scarcity in Southern Africa Technical Report: Second Order Water Scarcity in Southern Africa Prepared for: DDffIIDD Submitted February 2007 1 Second Order Water Scarcity in Southern Africa Disclaimer: “This report is an output from the Department for International Development (DfID) funded Engineering Knowledge and Research Programme (project no R8158, Second Order Water Scarcity). The views expressed are not necessarily those of DfID." Acknowledgements The authors would like to thank the organisations that made this research possible. The Department for International Development (DFID) that funded the Second Order Water Scarcity in Southern Africa Research Project and the Jack Wright Trust that provided a travel award for the researcher in Zambia. A special thank you also goes to the participants in the research, the people of Zambia and South Africa, the represented organisations and groups, for their generosity in sharing their knowledge, time and experiences. Authors Introduction: Dr Julie Trottier Zambia Case Study: Paxina Chileshe Research Director – Dr Julie Trottier South Africa Case Study: Chapter 9: Dr Zoë Wilson, Eleanor Hazell with general project research assistance from Chitonge Horman, Amanda Khan, Emeka Osuigwe, Horacio Zandamela Research Director – Dr Julie Trottier Chapter 10: Dr Zoë Wilson, Horacio Zandamela with general project research assistance from Eleanor Hazell, Chitonge Horman, Amanda Khan, Emeka Osuigwe, and principal advisor, Patrick Bond Research Director – Dr Julie Trottier Chapter 11: Dr Zoë Wilson with Kea Gordon, Eleanor Hazell and Karen Peters with general project support: Chitonge Horman, Mary Galvin, Amanda Khan, Emeka Osuigwe, Horacio Zandamela Research Director – Dr Julie Trottier Chapter 12: Karen Peters, Dr J. -

New Species of Congoglanis (Siluriformes: Amphiliidae) from the Southern Congo River Basin
New Species of Congoglanis (Siluriformes: Amphiliidae) from the Southern Congo River Basin Richard P. Vari1, Carl J. Ferraris, Jr.2, and Paul H. Skelton3 Copeia 2012, No. 4, 626–630 New Species of Congoglanis (Siluriformes: Amphiliidae) from the Southern Congo River Basin Richard P. Vari1, Carl J. Ferraris, Jr.2, and Paul H. Skelton3 A new species of catfish of the subfamily Doumeinae, of the African family Amphiliidae, was discovered from the Kasai River system in northeastern Angola and given the name Congoglanis howesi. The new species exhibits a combination of proportional body measurements that readily distinguishes it from all congeners. This brings to four the number of species of Congoglanis, all of which are endemic to the Congo River basin. ECENT analyses of catfishes of the subfamily Congoglanis howesi, new species Doumeinae of the African family Amphiliidae Figures 1, 2; Table 1 R documented that the species-level diversity and Doumea alula, Poll, 1967:265, fig. 126 [in part, samples from morphological variation of some components of the Angola, Luachimo River, Luachimo rapids; habitat infor- subfamily were dramatically higher than previously mation; indigenous names]. suspected (Ferraris et al., 2010, 2011). One noteworthy discovery was that what had been thought to be Doumea Holotype.—MRAC 162332, 81 mm SL, Angola, Lunda Norte, alula not only encompassed three species, but also that Kasai River basin, Luachimo River, Luachimo rapids, 7u219S, they all lacked some characters considered diagnostic of 20u509E, in residual pools downstream of dam, A. de Barros the Doumeinae. Ferraris et al. (2011) assigned those Machado, E. Luna de Carvalho, and local fishers, 10 Congoglanis species to a new genus, , which they hypoth- February 1957. -

Main Document.Pdf (2.455Mb)
DOMESTIC WATER USE AND CONSERVATION PRACTICES AMONG THE HOUSEHOLDS OF KANSENSHI AND NDEKE RESIDENTIAL AREAS OF NDOLA CITY IN ZAMBIA By Vwambanji Namuwelu A Dissertation Submitted to the University of Zambia in Partial Fulfilment of the Requirements of the Degree of Master of Science in Environmental and Natural Resources Management THE UNIVERSITY OF ZAMBIA LUSAKA 2020 COPYRIGHT All rights reserved. No part of this dissertation may be reproduced or stored in any form or by any means without prior permission in writing from the author or the University of Zambia i DECLARATION I, VWAMBANJI NAMUWELU (2015131154), do hereby declare that this dissertation is my own work to the best of my knowledge and that it has never been produced or submitted for any degree, diploma or other qualification at the University of Zambia or indeed any other university for academic purposes. I further declare that all other works of people used in this research have been duly acknowledged. Vwambanji Namuwelu Signature: Date: ii CERTIFICATE OF APPROVAL This dissertation by VWAMBANJI NAMUWELU has been approved as fulfilling the partial requirements for the award of Master’s of Science Degree in Environmental and Natural Resources Management by the University of Zambia. ........................................ ................................... …………………….. Examiner 1 Signature Date ........................................ ................................... …………………….. Examiner 2 Signature Date ........................................ .................................. -

Fish Diversity, Community Structure, Feeding Ecology, and Fisheries of Lower Omo River and the Ethiopian Part of Lake Turkana, East Africa
Fish Diversity, Community Structure, Feeding Ecology, and Fisheries of Lower Omo River and the Ethiopian Part of Lake Turkana, East Africa Mulugeta Wakjira Addis Ababa University June 2016 Cover photos: Lower Omo River at Omorate town about 50 km upstream of the delta (upper photo); Lake Turkana from Ethiopian side (lower photo). © Mulugeta Wakjira and Abebe Getahun Fish diversity, Community structure, Feeding ecology, and Fisheries of lower Omo River and the Ethiopian part of Lake Turkana, East Africa Mulugeta Wakjira A Thesis Submitted to the Department of Zoological Sciences, Addis Ababa University, Presented in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology (Fisheries and Aquatic Sciences) June 2016 ADDIS ABABA UNIVERSITY SCHOOL OF GRADUATE PROGRAM This is to certify that the thesis prepared by Mulugeta Wakjira entitled, "Fish Diversity, Community Structure, Feeding Ecology, and Fisheries of lower Omo River and the Ethiopian part of Lake Turkana, East Africa", and submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biology (Fisheries and Aquatic Science) complies with the regulations of the university and meets the accepted standards with respect to originality and quality. Signed by the Examining Committee Examiner (external): Dr. Leo Nagelkerke Signature ____________ Date_________ Examiner (internal): Dr. Elias Dadebo Signature ____________ Date_________ Advisor: Dr. Abebe Getahun Signature ____________ Date__________ ____________________________________________________________ Chair of Department or Graduate Program Coordinator Abstract Ethiopia has a freshwater system in nine major drainage basins which fall into four ichthyofaunal provinces and one subprovince. Omo-Turkana Basin, spanning considerable geographic area in southwestern Ethiopia and northern Kenya, essentially consists of Omo River (also known as Omo-Gibe) and Lake Turkana. -

Ministry of Agriculture Food Security and Cooperatives
THE UNITED REPUBLIC OF TANZANIA MINISTRY OF AGRICULTURE FOOD SECURITY AND COOPERATIVES AGRICULTURAL SECTOR DEVELOPMENT PROGRAMME IRRIGATION DEVELOPMENT SUB-COMPONENT THE STRATEGIC ENVIRONMENTAL AND SOCIAL ASSESSMENT (SESA) FOR THE NATIONAL IRRIGATION MASTER PLAN (NIMP) AND THE NATIONAL IRRIGATION POLICY (NIP) FINAL SESA REPORT VOLUME II: MAIN REPORT 25TH APRIL 2011 SUBMITTED BY CONSULTANT: SMEC International Pty Limited P.O. Box 105866 Uganda Avenue/Mzinga Way Plot 191, Oyster Bay Dar es Salaam, Tanzania THE STRATEGIC ENVIRONMENTAL AND SOCIAL ASSESSMENT (SESA) FOR THE NATIONAL IRRIGATION MASTER PLAN (NIMP) AND THE NATIONAL IRRIGATION POLICY (NIP) VOLUME II: MAIN REPORT Prepared by: SMEC International Pty Limited For The Ministry of Agriculture Food Security and Cooperatives, Dar es Salaam, Tanzania SESA Team: Mr. Victor A. Gillespie, SMEC, Team Leader / ESIA – SESA Specialist Prof. Fredrick Mwanuzi, SMEC, Deputy Team Leader / ESIA – SESA Specialist Mr. Vernon Copeland, SMEC, Agronomist Ms. Janet Ishengoma, SMEC, Sociologist Dr. Reuben Kadigi, SMEC, Economist Eng. Stephen Kamugisha, SMEC, Irrigation Engineer Dr. Japhet Kashaigili, SMEC, Hydrologist (General) Ms. Priscilla Madzonga, SMEC, Legal Specialist Prof. Pantaleo Munishi, SMEC, Ecologist Dr. Peter Hawkins, SMEC, Water Quality/Public Health Expert Mr. Rob van der Weert, SMEC, Hydrologist (Climate Specialist) The Strategic Environmental and Social Assessment (SESA) for the National Irrigation Master Plan (NIMP) and the National Irrigation Policy (NIP) 25th April 2011 Page | i ACKNOWLEDGEMENTS The Project Team acknowledges with appreciation the Key Stakeholders who provided their time and shared their knowledge. The Project Team also acknowledges with appreciation the hard work and insight of Deputy Permanent Secretary Eng. M. Futakamba (Former Director of the Division of Irrigation and Technical Services (DITS); Acting Director of DITS, Eng. -

5Th Indo-Pacific Fish Conference
)tn Judo - Pacifi~ Fish Conference oun a - e II denia ( vernb ~ 3 - t 1997 A ST ACTS Organized by Under the aegis of L'Institut français Société de recherche scientifique Française pour le développement d'Ichtyologie en coopération ' FI Fish Conference Nouméa - New Caledonia November 3 - 8 th, 1997 ABSTRACTS LATE ARRIVAL ZOOLOGICAL CATALOG OF AUSTRALIAN FISHES HOESE D.F., PAXTON J. & G. ALLEN Australian Museum, Sydney, Australia Currently over 4000 species of fishes are known from Australia. An analysis ofdistribution patterns of 3800 species is presented. Over 20% of the species are endemic to Australia, with endemic species occuiring primarily in southern Australia. There is also a small component of the fauna which is found only in the southwestern Pacific (New Caledonia, Lord Howe Island, Norfolk Island and New Zealand). The majority of the other species are widely distributed in the western Pacific Ocean. AGE AND GROWTH OF TROPICAL TUNAS FROM THE WESTERN CENTRAL PACIFIC OCEAN, AS INDICATED BY DAILY GROWm INCREMENTS AND TAGGING DATA. LEROY B. South Pacific Commission, Nouméa, New Caledonia The Oceanic Fisheries Programme of the South Pacific Commission is currently pursuing a research project on age and growth of two tropical tuna species, yellowfm tuna (Thunnus albacares) and bigeye tuna (Thunnus obesus). The daily periodicity of microincrements forrned with the sagittal otoliths of these two spceies has been validated by oxytetracycline marking in previous studies. These validation studies have come from fishes within three regions of the Pacific (eastem, central and western tropical Pacific). Otolith microincrements are counted along transverse section with a light microscope. -

Siluriformes: Amphiliidae) from the Congo River Basin, the Sister-Group to All Other Genera of the Doumeinae, with the Description of Two New Species
A New Genus of African Loach Catfish (Siluriformes: Amphiliidae) from the Congo River Basin, the Sister-Group to All Other Genera of the Doumeinae, with the Description of Two New Species Carl J. Ferraris, Jr.1, Richard P. Vari2, and Paul H. Skelton3 Copeia 2011, No. 4, 477–489 A New Genus of African Loach Catfish (Siluriformes: Amphiliidae) from the Congo River Basin, the Sister-Group to All Other Genera of the Doumeinae, with the Description of Two New Species Carl J. Ferraris, Jr.1, Richard P. Vari2, and Paul H. Skelton3 Congo River basin catfishes previously identified as Doumea alula (Amphiliidae, Doumeinae) were found to include three species that belong not to the genus Doumea but are, instead, the sister-group to a clade formed by all remaining Doumeinae. The species are assigned to a new genus, Congoglanis. Characters delimiting the Doumeinae and the clade consisting of all members of the subfamily except Congoglanis are detailed. Congoglanis alula is distributed throughout much of the Congo River basin; C. inga, new species, is known only from the lower Congo River in the vicinity of Inga Rapids; and C. sagitta, new species, occurs in the Lualaba River basin of Zambia in the southeastern portion of the Congo River system. ATFISHES of the subfamily Doumeinae of the Congoglanis, new genus Amphiliidae inhabit rapidly flowing rivers and Type species.—Congoglanis inga, new species C streams across a broad expanse of tropical Africa. Recent studies of the Doumeinae documented that the Diagnosis.—Congoglanis includes species that possess the species diversity and morphological variability within the following combination of characters unique within the subfamily are greater than previously suspected (Ferraris Amphiliidae: the caudal peduncle is relatively short and et al., 2010). -

Siluriformes, Amphiliidae) with the Descriptions of New Species from the Upper Sanaga River and Nyong River Basins
Species of the Doumea chappuisi Complex (Siluriformes, Amphiliidae) with the Descriptions of New Species from the Upper Sanaga River and Nyong River Basins Carl J. Ferraris, Jr.1, Paul Skelton2, and Richard P. Vari3 Copeia 2010, No. 4, 705–715 Species of the Doumea chappuisi Complex (Siluriformes, Amphiliidae) with the Descriptions of New Species from the Upper Sanaga River and Nyong River Basins Carl J. Ferraris, Jr.1, Paul Skelton2, and Richard P. Vari3 The Doumea chappuisi complex within the catfish family Amphiliidae is diagnosed on the form of the dorsolateral and ventrolateral processes of the vertebrae along the posterior portion of the body. Three species are recognized in the complex: Doumea chappuisi of the West African coastal river basins in Guinea, Guinea-Bissau, Cote d’Ivoire, and Liberia; D. reidi, new species, described herein from a portion of the upper Sanaga River in Nigeria; and D. stilicauda, new species, described herein from the Nyong River basin in Cameroon. Members of the complex are distinguished from each other on the basis of the overall body form, the caudal-peduncle length, the predorsal length, the head length, the degree of development of the pelvic fin in larger specimens, the anterior extent of the exposed vertebral processes along the ventral surface of the body, and details of the pigmentation pattern of the unbranched rays of the pectoral and pelvic fins. ATFISHES of the amphiliid genus Doumea range interesting specimen from the Nigerian portion of the upper across a major portion of Africa from Angola Sanaga River. While closely resembling other species of C through the Congo River basin and the Lower Doumea in most features, the vertebral ossifications of the Guinea region to Guinea-Bissau.