Aneura Pinguis Complex and Aneura Maxima in Poland
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identifikasi Keanekaragaman Marchantiophyta Di Kawasan Air Terjun Parangkikis Pagerwojo Tulungagung
Jurnal Biologi dan Pembelajarannya, Vol 6 No 2, Oktober 2019. Pp: 17-21 e-ISSN: 2406 – 8659 IDENTIFIKASI KEANEKARAGAMAN MARCHANTIOPHYTA DI KAWASAN AIR TERJUN PARANGKIKIS PAGERWOJO TULUNGAGUNG Repik Febriansah, Eni Setyowati*), Arbaul Fauziah Jurusan Tadris Biologi, Fakultas Tarbiyah dan Ilmu Keguruan, IAIN Tulungagung Jalan Mayor Sujadi No. 46 Tulungagung *)Email: [email protected] Abstrak Penelitian ini bertujuan untuk mengkaji keanekaragaman jenis dari Divisi Marchantiophyta di Kawasan Air Terjun Parangkikis. Pengambilan sampel dilakukan pada bulan Desember 2018 hingga Maret 2019 dengan metode jelajah di sekitar Air Terjun Parangkikis Pagerwojo, Tulungagung. Identifikasi Marchantiophyta dilakukan di Laboratorium IPA Fakultas Tarbiyah dan Ilmu Keguruan IAIN Tulungagung. Hasil penelitian menunjukkan bahwa di kawasan Air Terjun Parangkikis terdapat dua kelas, yaitu Marchantiopsida dan Jungermanniopsida. Pada kelas Marchantiopsida hanya terdapat satu ordo, yaitu Marchantiales. Sedangkan pada kelas Jungermanniopsida meliputi tiga ordo yaitu Jungermanniales, Porellales, dan Pallviciniales. Kata kunci- Air Terjun Parangkikis, Keanekaragaman, Marchantiophyta PENDAHULUAN Tumbuhan lumut merupakan salah satu tumbuhan yang memiliki keanekaragaman cukup tinggi. Lumut merupakan kelompok tumbuhan yang berukuran kecil yang tempat tumbuhnya menempel pada berbagai substrat seperti pohon, serasah, kayu mati, kayu lapuk, tanah, maupun bebatuan. Lumut dapat tumbuh pada lingkungan lembab dengan penyinaran yang cukup [1]. Secara ekologis lumut berperan penting di dalam fungsi ekosistem. Tumbuhan lumut dapat digunakan sebagai bioindikator lingkungan yang menentukan lingkungan tersebut masih terjaga dengan baik atau sudah tereksploitasi [2]. Lumut hati dapat berfungsi sebagai bioakumulator logam berat [3] dan inhibitor pertumbuhan protozoa [4]. Air Terjun Parangkikis merupakan salah satu daerah pegunungan di Desa Gambiran Kecamatan Pagerwojo Kabupaten Tulungagung yang kaya dengan berbagai jenis lumut. Namun, penelitian tumbuhan lumut di kawasan tersebut belum banyak dilakukan. -

Aquatic and Wet Marchantiophyta, Order Metzgeriales: Aneuraceae
Glime, J. M. 2021. Aquatic and Wet Marchantiophyta, Order Metzgeriales: Aneuraceae. Chapt. 1-11. In: Glime, J. M. Bryophyte 1-11-1 Ecology. Volume 4. Habitat and Role. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 11 April 2021 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 1-11: AQUATIC AND WET MARCHANTIOPHYTA, ORDER METZGERIALES: ANEURACEAE TABLE OF CONTENTS SUBCLASS METZGERIIDAE ........................................................................................................................................... 1-11-2 Order Metzgeriales............................................................................................................................................................... 1-11-2 Aneuraceae ................................................................................................................................................................... 1-11-2 Aneura .......................................................................................................................................................................... 1-11-2 Aneura maxima ............................................................................................................................................................ 1-11-2 Aneura mirabilis .......................................................................................................................................................... 1-11-7 Aneura pinguis .......................................................................................................................................................... -

Functional Gene Losses Occur with Minimal Size Reduction in the Plastid Genome of the Parasitic Liverwort Aneura Mirabilis
Functional Gene Losses Occur with Minimal Size Reduction in the Plastid Genome of the Parasitic Liverwort Aneura mirabilis Norman J. Wickett,* Yan Zhang, S. Kellon Hansen,à Jessie M. Roper,à Jennifer V. Kuehl,§ Sheila A. Plock, Paul G. Wolf,k Claude W. dePamphilis, Jeffrey L. Boore,§ and Bernard Goffinetà *Department of Ecology and Evolutionary Biology, University of Connecticut; Department of Biology, Penn State University; àGenome Project Solutions, Hercules, California; §Department of Energy Joint Genome Institute and University of California Lawrence Berkeley National Laboratory, Walnut Creek, California; and kDepartment of Biology, Utah State University Aneura mirabilis is a parasitic liverwort that exploits an existing mycorrhizal association between a basidiomycete and a host tree. This unusual liverwort is the only known parasitic seedless land plant with a completely nonphotosynthetic life history. The complete plastid genome of A. mirabilis was sequenced to examine the effect of its nonphotosynthetic life history on plastid genome content. Using a partial genomic fosmid library approach, the genome was sequenced and shown to be 108,007 bp with a structure typical of green plant plastids. Comparisons were made with the plastid genome of Marchantia polymorpha, the only other liverwort plastid sequence available. All ndh genes are either absent or pseudogenes. Five of 15 psb genes are pseudogenes, as are 2 of 6 psa genes and 2 of 6 pet genes. Pseudogenes of cysA, cysT, ccsA, and ycf3 were also detected. The remaining complement of genes present in M. polymorpha is present in the plastid of A. mirabilis with intact open reading frames. All pseudogenes and gene losses co-occur with losses detected in the plastid of the parasitic angiosperm Epifagus virginiana, though the latter has functional gene losses not found in A. -

A Taxonomic Revision of Aneuraceae (Marchantiophyta) from Eastern Africa with an Interactive Identification Key
cryptogamie Bryologie 2019 ● 41 ● 2 DIRECTEUR DE LA PUBLICATION : Bruno David, Président du Muséum national d’Histoire naturelle RÉDACTEURS EN CHEF / EDITORS-IN-CHIEF : Denis LAMY, Michelle Price ASSISTANTS DE RÉDACTION / ASSISTANT EDITORS : Marianne SALAÜN ([email protected]) MISE EN PAGE / PAGE LAYOUT : Marianne SALAÜN RÉDACTEURS ASSOCIÉS / ASSOCIATE EDITORS Biologie moléculaire et phylogénie / Molecular biology and phylogeny Bernard GOFFINET Department of Ecology and Evolutionary Biology, University of Connecticut (United States) Mousses d’Europe / European mosses Isabel DRAPER Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) Francisco LARA GARCÍA Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) Mousses d’Afrique et d’Antarctique / African and Antarctic mosses Rysiek OCHYRA Laboratory of Bryology, Institute of Botany, Polish Academy of Sciences, Krakow (Pologne) Bryophytes d’Asie / Asian bryophytes Rui-Liang ZHU School of Life Science, East China Normal University, Shanghai (China) Bioindication / Biomonitoring Franck-Olivier DENAYER Faculté des Sciences Pharmaceutiques et Biologiques de Lille, Laboratoire de Botanique et de Cryptogamie, Lille (France) Écologie des bryophytes / Ecology of bryophyte Nagore GARCÍA MEDINA Department of Biology (Botany), and Centro de Investigación en Biodiversidad y Cambio Global (CIBC-UAM), Universidad Autónoma de Madrid (Spain) COUVERTURE / COVER : From top left, to bottom right, by -

Divergence Times and the Evolution of Morphological Complexity in an Early Land Plant Lineage (Marchantiopsida) with a Slow Molecular Rate
Research Divergence times and the evolution of morphological complexity in an early land plant lineage (Marchantiopsida) with a slow molecular rate Juan Carlos Villarreal A.1,3,4, Barbara J. Crandall-Stotler2, Michelle L. Hart1, David G. Long1 and Laura L. Forrest1 1Royal Botanic Gardens Edinburgh, 20A Inverleith Row, Edinburgh, EH3 5LR, UK; 2Department of Plant Biology, Southern Illinois University, Carbondale, IL 62901, USA; 3Present address: Smithsonian Tropical Research Institute, Ancon, 0843-03092 Panama, Republic of Panama; 4Present address: Departement de Biologie, Universite Laval, Quebec, Canada G1V 0A6 Summary Authors for correspondence: We present a complete generic-level phylogeny of the complex thalloid liverworts, a lineage Juan Carlos Villarreal A that includes the model system Marchantia polymorpha. The complex thalloids are remark- Tel: +1418 656 3180 able for their slow rate of molecular evolution and for being the only extant plant lineage to Email: [email protected] differentiate gas exchange tissues in the gametophyte generation. We estimated the diver- Laura L. Forrest gence times and analyzed the evolutionary trends of morphological traits, including air cham- Tel: + 44(0) 131248 2952 bers, rhizoids and specialized reproductive structures. Email: [email protected] A multilocus dataset was analyzed using maximum likelihood and Bayesian approaches. Received: 29 June 2015 Relative rates were estimated using local clocks. Accepted: 15 September 2015 Our phylogeny cements the early branching in complex thalloids. Marchantia is supported in one of the earliest divergent lineages. The rate of evolution in organellar loci is slower than New Phytologist (2015) for other liverwort lineages, except for two annual lineages. -

On the Phylogeny and Taxonomy of Pallaviciniales
Arctoa (2015) 24: 98-123 doi: 10.15298/arctoa.24.12 ON THE PHYLOGENY AND TAXONOMY OF PALLAVICINIALES (MARCHANTIOPHYTA), WITH OVERVIEW OF RUSSIAN SPECIES ФИЛОГЕНИЯ И ТАКСОНОМИЯ ПОРЯДКА PALLAVICINIALES (MARCHANTIOPHYTA) С ОБЗОРОМ РОССИЙСКИХ ВИДОВ YURY S. MAMONTOV1,2, NADEZHDA A. KONSTANTINOVA3, ANNA A. VILNET3 & VADIM A. BAKALIN4,5 ЮРИЙ С. МАМОНТОВ1,2, НАДЕЖДА А. КОНСТАНТИНОВА3, АННА А. ВИЛЬНЕТ3, ВАДИМ А. БАКАЛИН4,5 Abstract Integrative analysis of expanded sampling of Pallaviciniales revealed the heterogeneity of Moercki- aceae. The new family Cordaeaceae Mamontov, Konstant., Vilnet & Bakalin is described based on morphology and molecular phylogenetic data. It includes one genus Cordaea Nees with two species, C. flotoviana (= Moerckia flotoviana), the type of the genus, and C. erimona (Steph.) Mamontov, Konstant., Vilnet & Bakalin comb. nov. Descriptions and illustrations of all species of the order known from Russia including newly reported Pallavicinia subciliata and provisional P. levieri are provided. Identification key for Pallaviciniales known from Russia and adjacent areas is given. Резюме В результате комплексного молекулярно-генетического и сравнительно-морфологического анализа расширенной выборки порядка Pallaviciniales выявлена гетерогенность сем. Moercki- aceae. Из него выделено новое семейство Cordaeaceae Mamontov, Konstant., Vilnet & Bakalin, включающее один род Cordaea Nees и два вида, C. flotoviana Nees (тип рода) и C. erimona (Steph.) Mamontov, Konstant., Vilnet & Bakalin comb. nov. Приведен ключ для определения видов порядка, встречающихся в России и на прилегающих территориях, даны описания и иллюстрации известных в России видов порядка, включая впервые выявленную для страны Pallavicinia subciliata, а также провизорно приводимую P. levieri, обнаруженную в республике Корея. KEYWORDS: Pallaviciniales, molecular phylogeny, taxonomy, Moerckiaceae, Cordaeaceae, Russia INTRODUCTION aration” of Moerckia that “supports Schuster’s (1992) Pallaviciniales W. -

Article ISSN 1179-3163 (Online Edition)
Phytotaxa 63: 21–68 (2012) ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2012 Magnolia Press Article ISSN 1179-3163 (online edition) Early Land Plants Today: Index of Liverworts & Hornworts 2009–2010 LARS SÖDERSTRÖM1, ANDERS HAGBORG2, MARSHALL R. CROSBY3 & MATT VON KONRAT2 1 Department of Biology, Norwegian University of Science and Technology, N-7491, Trondheim, Norway; [email protected] 2 Department of Botany, The Field Museum, 1400 South Lake Shore Drive, Chicago, IL 60605–2496, U.S.A.;[email protected], [email protected] 3 Missouri Botanical Garden, P. O. Box 299, St. Louis, MO 63166–0299 U.S.A.; [email protected] Abstract A widely accessible list of known plant species is a fundamental requirement for plant conservation and has vast applications. An index of published names of liverworts and hornworts between 2009 and 2010 is provided as part of a continued effort in working toward producing a world checklist of this group. Included in the list are also names overlooked by earlier indices. The list includes 30 higher taxa, 250 species, 52 infraspecific taxa, 31 autonyms, and two fossils for 2009 and 2010. A number of taxa not covered by the earlier indices for 2000-2008 are also included. Key words: Liverworts, hornworts, index, nomenclature Introduction Under the auspices of the Early Land Plants Today project, there has been a strong community-driven effort attempting to address the critical need to synthesize the vast nomenclatural, taxonomic and global distributional data for liverworts (Marchantiophyta) and hornworts (Anthocerotophyta) (von Konrat et al. 2010a). These endeavours are critical in providing the foundation to develop a working checklist of liverworts and hornworts worldwide; the first version is projected to be published in 2012. -

Microclimate Fluctuation Correlated with Beta Diversity of Epiphyllous Bryophyte Communities
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title Adaptive Traits and Community Assembly of Epiphyllous Bryophytes Permalink https://escholarship.org/uc/item/02t0k1qh Author Kraichak, Ekaphan Publication Date 2013 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California Adaptive Traits and Community Assembly of Epiphyllous Bryophytes By Ekaphan Kraichak A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Integrative Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Brent D. Mishler, Chair Professor David D. Ackerly Professor Katherine N. Suding Spring 2013 Abstract Adaptive Traits and Community Assembly of Epiphyllous Bryophytes By Ekaphan Kraichak Doctor of Philosophy in Integrative Biology University of California, Berkeley Professor Brent D. Mishler, Chair Leaf surfaces of tropical vascular plants provide homes for diverse groups of organisms, including epiphyllous (leaf-colonizing) bryophytes. Each leaf harbors a temporally and spatially discrete community of organisms, providing an excellent system for answering some of the most fundamental questions in ecology and evolution. In this dissertation, I investigated two main aspects of epiphyllous bryophyte biology: 1) adaptive traits of bryophytes to living on the leaf surface, and 2) community assembly of epiphyllous bryophytes in space (between-hosts) and time (succession). For the first part, I used published trait data and phylogeny of liverworts in family Lejeuneaceae to demonstrate that only the production of asexual propagules appeared to evolve in response to living on the leaf surface, while other hypothesized traits did not have correlated evolution with epiphylly. The second portion dealt with the assembly of communities among different host types. -

Wikstrom2009chap13.Pdf
Liverworts (Marchantiophyta) Niklas Wikströma,*, Xiaolan He-Nygrénb, and our understanding of phylogenetic relationships among A. Jonathan Shawc major lineages and the origin and divergence times of aDepartment of Systematic Botany, Evolutionary Biology Centre, those lineages. Norbyvägen 18D, Uppsala University, Norbyvägen 18D 75236, Altogether, liverworts (Phylum Marchantiophyta) b Uppsala, Sweden; Botanical Museum, Finnish Museum of Natural comprise an estimated 5000–8000 living species (8, 9). History, University of Helsinki, P.O. Box 7, 00014 Helsinki, Finland; Early and alternative classiA cations for these taxa have cDepartment of Biology, Duke University, Durham, NC 27708, USA *To whom correspondence should be addressed (niklas.wikstrom@ been numerous [reviewed by Schuster ( 10)], but the ebc.uu.se) arrangement of terminal taxa (species, genera) into lar- ger groups (e.g., families and orders) based on morpho- logical criteria alone began in the 1960s and 1970s with Abstract the work of Schuster (8, 10, 11) and Schljakov (12, 13), and culminated by the turn of the millenium with the work Liverworts (Phylum Marchantiophyta) include 5000–8000 of Crandall-Stotler and Stotler (14). 7 ree morphological species. Phylogenetic analyses divide liverworts into types of plant bodies (gametophytes) have generally been Haplomitriopsida, Marchantiopsida, and Jungerman- recognized and used in liverwort classiA cations: “com- niopsida. Complex thalloids are grouped with Blasiales in plex thalloids” including ~6% of extant species diversity Marchantiopsida, and leafy liverworts are grouped with and with a thalloid gametophyte that is organized into Metzgeriidae and Pelliidae in Jungermanniopsida. The distinct layers; “leafy liverworts”, by far the most speci- timetree shows an early Devonian (408 million years ago, ose group, including ~86% of extant species diversity and Ma) origin for extant liverworts. -
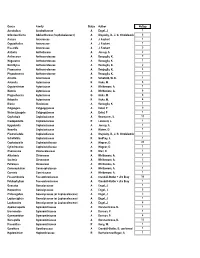
Family Status Author Nospp Acrobolbus Acrobolbaceae a Engel, J
Genus Family Status Author NoSpp Acrobolbus Acrobolbaceae A Engel, J. 1 Odontoschisma Adelanthaceae (Cephaloziaceae?) A Krayesky, D., J. G. Chmielewski 6 Aneura Aneuraceae A J. Faubert 1 Cryptothallus Aneuraceae A J. Faubert 1 Riccardia Aneuraceae A J. Faubert 5 Anthelia Antheliaceae A Jessup, S. 2 Anthoceros Anthocerotaceae A Renzaglia, K. 7 Megaceros Anthocerotaceae A Renzaglia, K. 1 Notothylas Anthocerotaceae A Renzaglia, K. 2 Phaeoceros Anthocerotaceae A Renzaglia, K. 4 Phymatoceros Anthocerotaceae A Renzaglia, K. 1 Arnellia Arnelliaceae R Schofield, W. B. 1 Asterella Aytoniaceae R Hicks, M. 8 Cryptomitrium Aytoniaceae A Whittemore, A. 1 Mannia Aytoniaceae A Whittemore, A. 6 Plagiochasma Aytoniaceae G Hicks, M. 6 Reboulia Aytoniaceae R Hicks, M. 4 Blasia Blasiaceae A Renzaglia, K. 1 Calypogeia Calypogejaceae A Eckel, P 9 Metacalypogeia Calypogejaceae A Eckel, P 1 Cephalozia Cephaloziaceae A Newmaster, S. 11 Cladopodiella Cephaloziaceae R Leonardi, L. 1 Hygrobiella Cephaloziaceae A Jessup, S. 1 Nowellia Cephaloziaceae A Waters, D. 1 Pleurocladula Cephaloziaceae A Krayesky, D., J. G. Chmielewski 1 Schofieldia Cephaloziaceae R Godfrey, J. 1 Cephaloziella Cephaloziellaceae A Wagner, D. 21 Cylindrocolea Cephaloziellaceae A Wagner, D. 1 Chonecolea Chonecoleaceae R Breil, D. 1 Athalamia Cleveaceae A Whittemore, A. 1 Sauteria Cleveaceae A Whittemore, A. 2 Peltolepis Cleveaceae A Whittemore, A. 1 Conocephalum Conocephalaceae A Whittemore, A. 1 Corsinia Corsiniaceae A Whittemore, A. 1 Fossombronia Fossombroniaceae A Crandall-Stotler + Jim Bray 10 Petalophyllum Fossombroniaceae A Crandall-Stotler + Jim Bray 1 Geocalyx Geocalycaceae A Engel, J. 1 Harpanthus Geocalycaceae A Engel, J. 3 Chiloscyphus Geocalycaceae (or Lophocoleaceae) A Engel, J. 3 Leptoscyphus Geocalycaceae (or Lophocoleaceae) A Engel, J. -

Article ISSN 2381-9685 (Online Edition)
Bry. Div. Evo. 043 (1): 284–306 ISSN 2381-9677 (print edition) DIVERSITY & https://www.mapress.com/j/bde BRYOPHYTEEVOLUTION Copyright © 2021 Magnolia Press Article ISSN 2381-9685 (online edition) https://doi.org/10.11646/bde.43.1.20 Advances in understanding of mycorrhizal-like associations in bryophytes SILVIA PRESSEL1*, MARTIN I. BIDARTONDO2, KATIE J. FIELD3 & JEFFREY G. DUCKETT1 1Life Sciences Department, The Natural History Museum, Cromwell Road, London SW7 5BD, UK; �[email protected]; https://orcid.org/0000-0001-9652-6338 �[email protected]; https://orcid.org/0000-0001-7101-6673 2Imperial College London and Royal Botanic Gardens, Kew TW9 3DS, UK; �[email protected]; https://orcid.org/0000-0003-3172-3036 3 Department of Animal and Plant Sciences, University of Sheffield, Sheffield, S10 2TN, UK; �[email protected]; https://orcid.org/0000-0002-5196-2360 * Corresponding author Abstract Mutually beneficial associations between plants and soil fungi, mycorrhizas, are one of the most important terrestrial symbioses. These partnerships are thought to have propelled plant terrestrialisation some 500 million years ago and today they play major roles in ecosystem functioning. It has long been known that bryophytes harbour, in their living tissues, fungal symbionts, recently identified as belonging to the three mycorrhizal fungal lineages Glomeromycotina, Ascomycota and Basidiomycota. Latest advances in understanding of fungal associations in bryophytes have been largely driven by the discovery, nearly a decade ago, that early divergent liverwort clades, including the most basal Haplomitriopsida, and some hornworts, engage with a wider repertoire of fungal symbionts than previously thought, including endogonaceous members of the ancient sub-phylum Mucoromycotina. -

Differentiation and Genetic Variability of Three Cryptic Species Within the Aneura Pinguis Complex (Jungermanniidae, Marchantiophyta)
Cryptogamie, Bryologie, 2016, 37 (1): 3-18 © 2016 Adac. Tous droits réservés Differentiation and genetic variability of three cryptic species within the Aneura pinguis complex (Jungermanniidae, Marchantiophyta) Alina Bączkiewicz* & Katarzyna Buczkowska Department of Genetics, a. Mickiewicz university, umultowska 89, 61-614 Poznań, Poland ABSTRACT – 1652 individuals of aneura pinguis from Poland were surveyed for variation in 12 putative gene loci. Based on isozyme data, we distinguished three cryptic species. No evidence for gene flow between these species was found. To date, no qualitative morphological characters are available, which would allow delimitation of the cryptic species of a. pinguis. Hence, these species are not formally described, but assigned as cryptic species A, B, and C. The mean genetic distance (D) between them is 1.3393. The highest genetic variation within populations (Hs) was found in species A, and the lowest in species B. Individual species of a. pinguis differ in their habitat preferences. Species A is the most common, it occurs mostly in the Western Carpathians, grows mainly on calcareous rocks and humus. Species B is the most frequent in the Eastern Carpathians on clay soil. Species C is the rarest, it can be found both in lowlands and mountains, but mainly in lowlands and on various substrata. All studied cryptic species occur partly sympatrically. Liverworts / Aneura pinguis / cryptic species / allozyme / cpDNA-trnL / genetic variation / geographic distribution / ecological preferences. RéSuMé – Différentiation and variabilité génétique de trios espèces cryptiques dans le complexe Aneura pinguis (Jungermanniidae, Marchantiophyta). Des investigations portant sur 12 loci enzymatiques ont été menées sur une population de 1652 individus d’aneura pinguis collectés en Pologne.