Studies on Range Expansion, Predation Pressure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Malacological Society of London
ACKNOWLEDGMENTS This meeting was made possible due to generous contributions from the following individuals and organizations: Unitas Malacologica The program committee: The American Malacological Society Lynn Bonomo, Samantha Donohoo, The Western Society of Malacologists Kelly Larkin, Emily Otstott, Lisa Paggeot David and Dixie Lindberg California Academy of Sciences Andrew Jepsen, Nick Colin The Company of Biologists. Robert Sussman, Allan Tina The American Genetics Association. Meg Burke, Katherine Piatek The Malacological Society of London The organizing committee: Pat Krug, David Lindberg, Julia Sigwart and Ellen Strong THE MALACOLOGICAL SOCIETY OF LONDON 1 SCHEDULE SUNDAY 11 AUGUST, 2019 (Asilomar Conference Center, Pacific Grove, CA) 2:00-6:00 pm Registration - Merrill Hall 10:30 am-12:00 pm Unitas Malacologica Council Meeting - Merrill Hall 1:30-3:30 pm Western Society of Malacologists Council Meeting Merrill Hall 3:30-5:30 American Malacological Society Council Meeting Merrill Hall MONDAY 12 AUGUST, 2019 (Asilomar Conference Center, Pacific Grove, CA) 7:30-8:30 am Breakfast - Crocker Dining Hall 8:30-11:30 Registration - Merrill Hall 8:30 am Welcome and Opening Session –Terry Gosliner - Merrill Hall Plenary Session: The Future of Molluscan Research - Merrill Hall 9:00 am - Genomics and the Future of Tropical Marine Ecosystems - Mónica Medina, Pennsylvania State University 9:45 am - Our New Understanding of Dead-shell Assemblages: A Powerful Tool for Deciphering Human Impacts - Sue Kidwell, University of Chicago 2 10:30-10:45 -

Taxonomic Revision of the Cretan Fauna of the Genus Temnothorax Mayr, 1861 (Hymenoptera: Formicidae), with Notes on the Endemism of Ant Fauna of Crete
ANNALES ZOOLOGICI (Warszawa), 2018, 68(4): 769-808 TAXONOMIC REVISION OF THE CRETAN FAUNA OF THE GENUS TEMNOTHORAX MAYR, 1861 (HYMENOPTERA: FORMICIDAE), WITH NOTES ON THE ENDEMISM OF ANT FAUNA OF CRETE SEBASTIAN SALATA1*, LECH BOROWIEC2, APOSTOLOS TRICHAS3 1Institute for Agricultural and Forest Environment, Polish Academy of Sciences, Bukowska 19, 60-809 Poznań, Poland; e-mail: [email protected] 2Department of Biodiversity and Evolutionary Taxonomy, University of Wrocław, Przybyszewskiego 65, 51-148 Wrocław, Poland; e-mail: [email protected] 3Natural History Museum of Crete, University of Crete, Greece; e-mail: [email protected] *Corresponding author Abstract.— We revise the Cretan species of the ant genus Temnothorax Mayr, 1861. Sixteen species are recognized, including seven new species which are possiblyendemic to Crete: T. crassistriatus sp. nov., T. daidalosi sp. nov., T. ikarosi sp. nov., T. incompletus sp. nov., T. minotaurosi sp. nov., T. proteii sp. nov., and T. variabilis sp. nov. A new synonymy is proposed, Temnothorax exilis (Emery, 1869) =Temnothorax specularis (Emery, 1916) syn. nov. An identification key to Cretan Temnothorax, based on worker caste is given. We provide a checklist of ant species described from Crete and discuss their status, distribution and endemism. Ë Key words.— Key, checklist, Myrmicinae, new species, Mediterranean Subregion, new synonymy INTRODUCTION 2000 mm in the high White Mountains range (Lefka Ori) (Grove et al. 1993). Temperature on mountains Crete is the fifth largest island in the Mediterranean seems to fall at a rate of about 6°C per 1000 m (Rack- ham & Moody 1996). Above 1600 m most of the precipi- Sea and the biggest island of Greece. -

Version of the Manuscript
Accepted Manuscript Antarctic and sub-Antarctic Nacella limpets reveal novel evolutionary charac- teristics of mitochondrial genomes in Patellogastropoda Juan D. Gaitán-Espitia, Claudio A. González-Wevar, Elie Poulin, Leyla Cardenas PII: S1055-7903(17)30583-3 DOI: https://doi.org/10.1016/j.ympev.2018.10.036 Reference: YMPEV 6324 To appear in: Molecular Phylogenetics and Evolution Received Date: 15 August 2017 Revised Date: 23 July 2018 Accepted Date: 30 October 2018 Please cite this article as: Gaitán-Espitia, J.D., González-Wevar, C.A., Poulin, E., Cardenas, L., Antarctic and sub- Antarctic Nacella limpets reveal novel evolutionary characteristics of mitochondrial genomes in Patellogastropoda, Molecular Phylogenetics and Evolution (2018), doi: https://doi.org/10.1016/j.ympev.2018.10.036 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Version: 23-07-2018 SHORT COMMUNICATION Running head: mitogenomes Nacella limpets Antarctic and sub-Antarctic Nacella limpets reveal novel evolutionary characteristics of mitochondrial genomes in Patellogastropoda Juan D. Gaitán-Espitia1,2,3*; Claudio A. González-Wevar4,5; Elie Poulin5 & Leyla Cardenas3 1 The Swire Institute of Marine Science and School of Biological Sciences, The University of Hong Kong, Pokfulam, Hong Kong, China 2 CSIRO Oceans and Atmosphere, GPO Box 1538, Hobart 7001, TAS, Australia. -

Crete (Chapter)
Greek Islands Crete (Chapter) Edition 7th Edition, March 2012 Pages 56 Page Range 256-311 PDF Coverage includes: Central Crete, Iraklio, Cretaquarium, Knossos, Arhanes, Zaros, Matala, Rethymno, Moni Arkadiou, Anogia, Mt Psiloritis, Spili, Plakias & around, Beaches Between Plakias & Agia Galini, Agia Galini, Western Crete, Hania & around, Samaria Gorge, Hora Sfakion & around, Frangokastello, Anopoli & Inner Sfakia, Sougia, Paleohora, Elafonisi, Gavdos Island, Kissamos-Kastelli & around, Eastern Crete, Lasithi Plateau, Agios Nikolaos & around, Mohlos, Sitia & around, Kato Zakros & Ancient Zakros, and Ierapetra & around. Useful Links: Having trouble viewing your file? Head to Lonely Planet Troubleshooting. Need more assistance? Head to the Help and Support page. Want to find more chapters? Head back to the Lonely Planet Shop. Want to hear fellow travellers’ tips and experiences? Lonely Planet’s Thorntree Community is waiting for you! © Lonely Planet Publications Pty Ltd. To make it easier for you to use, access to this chapter is not digitally restricted. In return, we think it’s fair to ask you to use it for personal, non-commercial purposes only. In other words, please don’t upload this chapter to a peer-to-peer site, mass email it to everyone you know, or resell it. See the terms and conditions on our site for a longer way of saying the above - ‘Do the right thing with our content. ©Lonely Planet Publications Pty Ltd Crete Why Go? Iraklio ............................ 261 Crete (Κρήτη) is in many respects the culmination of the Knossos ........................268 Greek experience. Nature here has been as prolifi c as Picas- Rethymno ..................... 274 so in his prime, creating a dramatic quilt of big-shouldered Anogia ......................... -
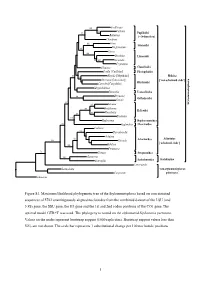
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia -

Crete 0 10 Miles
e# 0 20 km Crete 0 10 miles 36º N Archaeological Museum Marvel at treasures from ELEVATION ancient worlds 2000m 1500m Hania’s Old Town Rethymno’s Old Quarter Stroll the charming Experience the romance of 1000m Iraklio Wine Country Spinalonga Island 500m Venetian Harbour a Renaissance town SEA OF CRETE Sip Crete’s top Visit the leper colony turned 0 vintages tourist attraction Cape Spatha ä# Diktynna Moni Arkadiou Palace of Knossos Cape Rodopos Meditate on beauty and Walk in the footsteps of Vouxa Peninsula Gulf of the Minoans Gramvousa Moni Hania Stavros Moni Iannou Eremiti tragic history Islets Bay of #\ ä# Gonias Ü# Moni Governotou Bay of Kalathas Ü# GramvousaKissamos Ü# Vaï Beach Peninsula #\ Akrotiri Moni Agias Triadas Kissamos Kolymbari Peninsula #– Kick back under palm Falasarna #\ #\ #\ (Kastelli) Souda Bay trees #\ Platanias Hania #\ #\ #\ Voukolies Souda Cape Drapano Platanos ä# Cape Polyrrina Drapano Panormo Stavros Dia HANIA Peninsula #\ #\ Bali Almyros Bay #\ Rethymno Iraklio 33 Vryses #\ #\ Cape Agios Samaria Perama Moni Agia Omalos #\ Bay Iraklio Ioannis Cape #\ Gorge Georgioupolis Sideros Irini #\ Margarites #\ Axos ^# #– Hrysoskalitissas #\ National #\ Hersonisos Park Lake Episkopi Moni Ü# ä# Eleftherna ä# #\ Spinalonga Island Ü# Kandanos Lefka Ori ä## Malia R #\ Knossos #\ ä# #÷ Kournas#\ Arkadiou Sfendoni # Elounda (2453m) Argyroupoli #\ Kolokytha Moni #\ #\ Elafonisi # Samaria Cave Anogia Malia #\ Vaï 3Gorg3e Neapoli Peninsula Toplou #\ RETHYMNO Imbros Mt Psiloritis #\ Ü# #\ Sougia #\ Hora # Spili R Arhanes Ancient -

(Gastropoda, Pulmonata, (A.J. Wagner, 1927
BASTERIA, 70:131-132, 2006 The genus Inchoatia (Gastropoda, Pulmonata, Clausiliidae) validated and threemistakes corrected E. Gittenberger National Museum of Natural History Naturalis, P.O. Box 95X7, NL 2300 RA Leiden, The Netherlands; [email protected] & D.R. uit de Weerd Academy ofNatural Sciences, 1900 Benjamin Franklin Parkway, PA19103 Philadelphia, USA; [email protected] The genericname Inchoatia is validated by the designationof a type species and three mistakes are corrected. Key words: Gastropoda, Pulmonata, Clausiliidae, Alopiinae, Inchoatia, Protalbinaria, A.J. Wagner Greece. By omitting a type species designation, Gittenberger & Uit de Weerd (2006: 65) have introduced Inchoatia To validate the hereadd that incorrectly gen. nov. name, we Inchoatia inchoata (O. Boettger, 1889) has to be considered the type species. The species that is now called Isabellaria parnassia (O. Boettger, 1888), was originally classified with Clausilia Draparnaud, 1805, so that contrary to Gittenberger & Uit de Weerd (2006: 65) the author has to be cited in parentheses. Gittenberger & Uit de Weerd (2006: 61) referred to Carinigera (C.) buresi (A.J. Wagner, from 1927). However, according to Hesse (1933: 224), A.J. Wagner's paper dates 1928. As 1928. Hesse's is a consequence, 1927has to be replaced by statement supported by Polinski who indicated that this final (1929: 19), paper, i.e. Wagner's publication, was published only a few weeks before his death on June 12th, 1928. Contra Nordsieck (2001: 20) we do not consider ProtalbinariaA. [J.] Wagner, 1923, a nomen nudum. A. [J.] Wagner (1923) published a description. The generic name was introduced withoutany included nominal species indeed, but before 1931, which implies that it is available (Art. -

Morphology, (Mollusca: Gastropoda Pulmonata)
BASTERIA, 52: 77-100, 1988 On morphology, function and taxonomic importance of the shell ribs in Clausiliidae (Mollusca: Gastropoda Pulmonata), with special reference to those in Albinaria Th.C.M. Kemperman & E. Gittenberger Systematic Zoology Section, Leiden University, c/o Rijksmuseum van Natuurlijke Historic, P.O. Box 9517, NL 2300 RA Leiden, The Netherlands In and other the shell hollow Clausiliidae, Urocoptidae a few groups ribs are not solid, but and and provided with complicated structures inside. These structures are described il- lustrated. Their be correlated with certain least within presence may a habitat, at a poly- Several of ribs and morphic species. physical consequences are suggested some are ex- perimentally tested. Especially shell weight and strength, surface water adhesion, shell permeabilityand warming up after insolation are dealt with. The possible adaptive value is discussed. Key words: Gastropoda Pulmonata, Clausiliidae, Albinaria, shell ribs, shell structure, shell formation, adaptation, taxonomy. Contents 1. Introduction 77 2. Material 78 2.1. Specieswith shells with hollow ribs 80 2.2. Species with shells with massive ribs 81 3. Morphologyofthe hollow ribs 81 3.1. The generalstructure 81 3.2. The septal particles 84 3.3. Chemical compositionofthe crystals 89 of 4. Development the ribs 89 5. Hollowribs and phylogeny 90 6. Hollowribs and habitat 91 7. and relevance ofshell ribs Physical consequences biological 94 7.1. Shell weight and strength 94 7.2. Surface water adhesion 95 7.3. Evaporationand shell permeability 97 7.4. Ribs and insolation 97 8. Acknowledgements 98 9. References 98 10. Samenvatting 99 1. Introduction Gastropod shells are often provided with ribs (costae), which are transverse eleva- on surface, different from the remains of former tions the shell e.g. -

Supplementary Material to the European Red List of Terrestrial Molluscs
Neubert, E., Seddon, M.B., Allen, D.J., Arrébola, J., Backeljau, T., Balashov, I., Bank, R., Cameron, R., de Frias Martins, A.M., De Mattia, W., Dedov, I., Duda, M., Falkner, G., Falkner, M., Fehér, Z., Gargominy, O., Georgiev, D., Giusti, F., Gómez Moliner, B.J., Groh, K., Ibáñez, M., Kappes, H., Manganelli, G., Martínez-Ortí, A., Nardi, G., Neiber, M.T., Páll-Gergely, B., Parmakelis, A., Prié, V., Reischütz, A., Reischütz, P.L., Rowson, B., Rüetschi, J., Slapnik, R., Son, M., Štamol, V., Teixeira, D., Triantis, K., Vardinoyannis, K., von Proschwitz, T. and Walther, F. 2019. Supplementary Material to the European Red List of terrestrial molluscs. Cambridge, UK: IUCN. Available at: https://portals.iucn.org/library/node/48439 Supplementary Material to the European Red List of terrestrial molluscs This document provides the Red List Category and Criteria for the European terrestrial mollusc species assessed on the IUCN European Red List. Data were compiled as part of the LIFE project, ‘Establishing a European Red List of Bryophytes, Pteridophytes, Saproxylic Beetles, Terrestrial Molluscs and Vascular Plants (LIFE European Red Lists; LIFE14 PRE/BE/000001)’. Includes data compiled through an earlier stage of the European Red List: Cuttelod, A., Seddon, M. and Neubert, E. 2011. European Red List of Non-marine Molluscs. Luxembourg: Publications Office of the European Union. An * indicates that a species was assessed for the EU 27 Member States, i.e., prior to the accession of Croatia in 2013. IUCN Red List status of European terrestrial mollusc -

Introduction Acknowledgements
10 11 Acknowledgements Introduction General geography of Greece Greece is a relatively small country, and with a surface area of 132,000 km2 it is only half as big as the UK. Encompassed, however, in this modest area, is a great diversity of habitats, exceeding many European countries of much larger size. For example, one can encounter in Epirus alpine areas complete with lush conifer forests, dramatic peaks and extensive snowfields that physiographically resemble Switzerland. On the other hand, some regions of the southern Aegean are closer to Africa than to Athens, and their climate and habitats reflect this proximity. Southeastern Crete for example, con- tains one of the few true European deserts, an area closely resembling certain hamma- da regions of the Middle East. Greece is a country of mountains and islands. The Pindos range, an extension of the Dinaric Alps, forms the backbone of peninsular Greece. A number of smaller mountains originate as spurs from this block, although some, including Mount Olympus, the highest mountain in Greece (2,917 m elevation) arise in relative isola- tion. A second major mountain block, the Rhodopes, located in Thrace, runs in a roughly east-west direction separating Greece and Bulgaria. The Peloponnese, a small- er peninsula in the south, is as mountainous as the mainland and encompasses several peaks exceeding 2,000 m in elevation. With the exception of a few large flat regions located mostly in Thessaly and Thrace, the country lacks extensive plains. Typically the mountains drop rather steeply into the sea and are generally flanked only by narrow coastal plains. -

Crete Bird Report 2000 and 2001
CRETE BIRD REPORT 2000-2001 WEATHER: 2000: Sunny and overcast in the first week in April with a hot southern wind in the west on 5th, then bright anda cold wind for the rest of April until 18th when there was rain and cold winds for 3 days. It was clear and settled from then onwards until the first week in May when there were strong winds and storms and large numbers of migrants on the south coast were reported. From the 9th May the weather was more settled. Strong winds reported on Ist June, which brought in Gulls including Audouin’s at Elounda. In the Autimn, late September and early October, there was some bad weather with gale force winds, driving rain and very little sunshine. Passage of birds was noted on 7th and 8th October. The rest of the month was settled with occasional brief showers. 2001: April began cold and wet with temperatures of 13° recorded in Plakias, on 3rd, and cold and wet, bright intervals and then heavy weather, which brought in migrants. Weather still cool and unsettled until 10th, but good birding resulted. Late April was warmer and humid with thunderstorms and Sahara sand, then sunny but persistent fresh blustery N W winds. During the last week of April the weather settled at last with temperatures of 27° and continued hot with strong northerly winds, then settling by 8th May when it became hot with cloudless skies. The Autumn was hot in September and October. The weather broke in early November with the winter rains arriving. -

Gastropoda Pulmonata: Clausiliidae
BASTERIA, 56: 159-161, 1992 The earliest name inAlbinaria Vest, 1867 (Gastropoda Pulmonata:Clausiliidae), clarified after two centuries E. Gittenberger & M. Schilthuizen Nationaal Natuurhistorisch Museum, P.O. Box 9517, NL 2300 RA Leiden The Netherlands Bulimus the earliest in the Albinaria corrugatus Bruguière, 1792, name speciose genus Vest, is the 1867, clarified by designation of a neotype. Key words: Gastropoda, Pulmonata, Clausiliidae, Albinaria, taxonomy, nomenclature, neotype. Bruguiere (1792: 354) describedBulimus corrugatus at length, referring to a text and illustrations published by "Martini [= Chemnitz]" (1786: 120, pi. 112 figs. 961, 962). The species was said to occur in Spain, and the Provence and Languedoc in France. However, a sinistral, clausiliid species that recalls this one conchologically in shape and size, sculpture on both the body whorl and the rest of the shell, and colour, does not occur in SW. Europe. Draparnaud (1801: 62; 1805: 70), referring to Bruguiere, mentionedthis species as Pupa corrugata and Clausilia corrugata, respectively. Michaud (1831: 54, 55) emphasized later on that Draparnaud had erroneously it from France. the of this remains reported Thus, formally type locality species unknown. Both Beck (1837: 91) and Nordsieck (1977: 297) considered Draparnaud's inter- pretation of Bruguiere's nominal taxon incorrect, without arguing why they did so. introduced Clausilia for the Beck (1837: 91) the name draparnaldi species figured by Draparnaud (1805: pi. 4 figs. 11, 12) and listed it next to Clausilia corrugata as another species. Nordsieck (1977: 302) listed “corrugata” Bruguiere, 1792, as a "nom. dub.", separate from corrugata Draparnaud, 1805, "non BRUGUIERE", and accepted draparnaldi Beck as the name for the latter taxon.