Appendix 1 Table A1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

<I>Palaeococcus Fuscipennis</I>
Folia biologica (Kraków), vol. 53 (2005), No 1-2 Ultrastructural Studies of the Ovary of Palaeococcus fuscipennis (Burmeister) (Insecta, Hemiptera, Coccinea: Monophlebidae)* Teresa SZKLARZEWICZ, Katarzyna KÊDRA and Sylwia NI¯NIK Accepted January 25, 2005 SZKLARZEWICZ T., KÊDRA K., NI¯NIK S. 2005. Ultrastructural studies of the ovary of Palaeococcus fuscipennis (Burmeister) (Insecta, Hemiptera, Coccinea: Monophlebidae). Folia biol. (Kraków) 53: 45-50. Ovaries of Palaeocoocus fuscipennis are composed of about 100 telotrophic ovarioles that are devoid of terminal filaments. In the ovariole a tropharium (=trophic chamber) and vitellarium can be distinguished. The tropharium contains 7 trophocytes. A single oocyte develops in the vitellarium. The oocyte is surrounded by follicular cells that do not undergo diversification into subpopulations. The obtained results are discussed in a phylogenetic context. Key words: Oogenesis, ovariole, phylogeny, scale insects, ultrastructure. Teresa SZKLARZEWICZ, Katarzyna KÊDRA, Sylwia NI¯NIK, Department of Systematic Zoology and Zoogeography, Institute of Zoology, Jagiellonian University, R. Ingardena 6, 30-060 Kraków, Poland. E: mail: [email protected] Scale insects (coccids, coccoids) are the most tive scale insects into two families, Ortheziidae specialized hemipterans. In view of the fact that and Margarodidae s. l. The latter has been subdi- they are chararacterized by numerous synapomor- vided into five subfamilies, i.e., the Margarodinae, phies, their monophyletic origin is widely ac- Monophlebinae, Coelostomidiinae, Xylococcinae cepted by entomologists (e.g., KOTEJA 1974 a, and Steingeliinae. While the monyphyly of the MILLER 1984, DANZIG 1986, FOLDI 1997, COOK family Ortheziidae is well documented (KOTEJA et al. 2002). Scale insects are usually divided into 1974 a, GULLAN &SJAARDA 2001, COOK et al. -

Zootaxa,Phylogeny and Higher Classification of the Scale Insects
Zootaxa 1668: 413–425 (2007) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2007 · Magnolia Press ISSN 1175-5334 (online edition) Phylogeny and higher classification of the scale insects (Hemiptera: Sternorrhyncha: Coccoidea)* P.J. GULLAN1 AND L.G. COOK2 1Department of Entomology, University of California, One Shields Avenue, Davis, CA 95616, U.S.A. E-mail: [email protected] 2School of Integrative Biology, The University of Queensland, Brisbane, Queensland 4072, Australia. Email: [email protected] *In: Zhang, Z.-Q. & Shear, W.A. (Eds) (2007) Linnaeus Tercentenary: Progress in Invertebrate Taxonomy. Zootaxa, 1668, 1–766. Table of contents Abstract . .413 Introduction . .413 A review of archaeococcoid classification and relationships . 416 A review of neococcoid classification and relationships . .420 Future directions . .421 Acknowledgements . .422 References . .422 Abstract The superfamily Coccoidea contains nearly 8000 species of plant-feeding hemipterans comprising up to 32 families divided traditionally into two informal groups, the archaeococcoids and the neococcoids. The neococcoids form a mono- phyletic group supported by both morphological and genetic data. In contrast, the monophyly of the archaeococcoids is uncertain and the higher level ranks within it have been controversial, particularly since the late Professor Jan Koteja introduced his multi-family classification for scale insects in 1974. Recent phylogenetic studies using molecular and morphological data support the recognition of up to 15 extant families of archaeococcoids, including 11 families for the former Margarodidae sensu lato, vindicating Koteja’s views. Archaeococcoids are represented better in the fossil record than neococcoids, and have an adequate record through the Tertiary and Cretaceous but almost no putative coccoid fos- sils are known from earlier. -

A Guide to the Ants of Sabangau
A Guide to the Ants of Sabangau The Orangutan Tropical Peatland Project November 2014 A Guide to the Ants of Sabangau All original text, layout and illustrations are by Stijn Schreven (e-mail: [email protected]), supple- mented by quotations (with permission) from taxonomic revisions or monographs by Donat Agosti, Barry Bolton, Wolfgang Dorow, Katsuyuki Eguchi, Shingo Hosoishi, John LaPolla, Bernhard Seifert and Philip Ward. The guide was edited by Mark Harrison and Nicholas Marchant. All microscopic photography is from Antbase.net and AntWeb.org, with additional images from Andrew Walmsley Photography, Erik Frank, Stijn Schreven and Thea Powell. The project was devised by Mark Harrison and Eric Perlett, developed by Eric Perlett, and coordinated in the field by Nicholas Marchant. Sample identification, taxonomic research and fieldwork was by Stijn Schreven, Eric Perlett, Benjamin Jarrett, Fransiskus Agus Harsanto, Ari Purwanto and Abdul Azis. Front cover photo: Workers of Polyrhachis (Myrma) sp., photographer: Erik Frank/ OuTrop. Back cover photo: Sabangau forest, photographer: Stijn Schreven/ OuTrop. © 2014, The Orangutan Tropical Peatland Project. All rights reserved. Email [email protected] Website www.outrop.com Citation: Schreven SJJ, Perlett E, Jarrett BJM, Harsanto FA, Purwanto A, Azis A, Marchant NC, Harrison ME (2014). A Guide to the Ants of Sabangau. The Orangutan Tropical Peatland Project, Palangka Raya, Indonesia. The views expressed in this report are those of the authors and do not necessarily represent those of OuTrop’s partners or sponsors. The Orangutan Tropical Peatland Project is registered in the UK as a non-profit organisation (Company No. 06761511) and is supported by the Orangutan Tropical Peatland Trust (UK Registered Charity No. -

The Entomofauna on Eucalyptus in Israel: a Review
EUROPEAN JOURNAL OF ENTOMOLOGYENTOMOLOGY ISSN (online): 1802-8829 Eur. J. Entomol. 116: 450–460, 2019 http://www.eje.cz doi: 10.14411/eje.2019.046 REVIEW The entomofauna on Eucalyptus in Israel: A review ZVI MENDEL and ALEX PROTASOV Department of Entomology, Institute of Plant Protection, Agricultural Research Organization, The Volcani Center, Rishon LeTzion 7528809, Israel; e-mails: [email protected], [email protected] Key words. Eucalyptus, Israel, invasive species, native species, insect pests, natural enemies Abstract. The fi rst successful Eucalyptus stands were planted in Israel in 1884. This tree genus, particularly E. camaldulensis, now covers approximately 11,000 ha and constitutes nearly 4% of all planted ornamental trees. Here we review and discuss the information available about indigenous and invasive species of insects that develop on Eucalyptus trees in Israel and the natural enemies of specifi c exotic insects of this tree. Sixty-two phytophagous species are recorded on this tree of which approximately 60% are indigenous. The largest group are the sap feeders, including both indigenous and invasive species, which are mostly found on irrigated trees, or in wetlands. The second largest group are wood feeders, polyphagous Coleoptera that form the dominant native group, developing in dying or dead wood. Most of the seventeen parasitoids associated with the ten invasive Eucalyptus-specifi c species were introduced as biocontrol agents in classical biological control projects. None of the polyphagous species recorded on Eucalyptus pose any threat to this tree. The most noxious invasive specifi c pests, the gall wasps (Eulophidae) and bronze bug (Thaumastocoris peregrinus), are well controlled by introduced parasitoids. -

American Museum Novitates
AMERICAN MUSEUM NOVITATES Number 3823, 80 pp. January 16, 2015 Diverse new scale insects (Hemiptera: Coccoidea) in amber from the Cretaceous and Eocene with a phylogenetic framework for fossil Coccoidea ISABELLE M. VEA1, 2 AND DAVID A. GRIMALDI2 ABSTRACT Coccoids are abundant and diverse in most amber deposits around the world, but largely as macropterous males. Based on a study of male coccoids in Lebanese amber (Early Cretaceous), Burmese amber (Albian-Cenomanian), Cambay amber from western India (Early Eocene), and Baltic amber (mid-Eocene), 16 new species, 11 new genera, and three new families are added to the coccoid fossil record: Apticoccidae, n. fam., based on Apticoccus Koteja and Azar, and includ- ing two new species A. fortis, n. sp., and A. longitenuis, n. sp.; the monotypic family Hodgsonicoc- cidae, n. fam., including Hodgsonicoccus patefactus, n. gen., n. sp.; Kozariidae, n. fam., including Kozarius achronus, n. gen., n. sp., and K. perpetuus, n. sp.; the irst occurrence of a Coccidae in Burmese amber, Rosahendersonia prisca, n. gen., n. sp.; the irst fossil record of a Margarodidae sensu stricto, Heteromargarodes hukamsinghi, n. sp.; a peculiar Diaspididae in Indian amber, Nor- markicoccus cambayae, n. gen., n. sp.; a Pityococcidae from Baltic amber, Pityococcus monilifor- malis, n. sp., two Pseudococcidae in Lebanese and Burmese ambers, Williamsicoccus megalops, n. gen., n. sp., and Gilderius eukrinops, n. gen., n. sp.; an Early Cretaceous Weitschatidae, Pseudo- weitschatus audebertis, n. gen., n. sp.; four genera considered incertae sedis, Alacrena peculiaris, n. gen., n. sp., Magnilens glaesaria, n. gen., n. sp., and Pedicellicoccus marginatus, n. gen., n. sp., and Xiphos vani, n. -

Crazy Ant (Paratrechina Sp
Midsouth Entomologist 3: 44–47 ISSN: 1936-6019 www.midsouthentomologist.org.msstate.edu Report The Invasive Rasberry Crazy Ant, Nylanderia sp. near pubens (Hymenoptera: Formicidae), Reported from Mississippi MacGown. J.1* and B. Layton1 Department of Entomology & Plant Pathology, Mississippi State University, Mississippi State, MS, 39762 *Corresponding Author: [email protected] Received: 18-XII-2009 Accepted: 23-XII-2009 Introduction The genus Nylanderia (Hymenoptera: Formicidae: Lasiini) currently includes 134 valid species and subspecies worldwide (LaPolla et al. 2010). Fifteen described species, including nine native and six introduced species, have been collected in the continental United States (Bolton et al. 2006, Trager 1984, Meyers 2008). Formerly, Nylanderia was considered to be a subgenus of Paratrechina and was only recently elevated to the generic level by LaPolla et al. (2010); consequently, it is referred to as Paratrechina in most of the literature. In 2002, large populations of an unknown Nylanderia species were discovered in Houston, Texas. After examination by taxonomic specialists, these ants were determined to be similar to Nylanderia pubens, the hairy crazy ant (Meyers 2008), known to occur in the Neotropical Region and in Florida (Wetterer and Keularts 2008), and similar to N. fulva (Mayr), which is native to South America (Meyers 2008). However, due to taxonomic uncertainty, this species has not been definitively identified as N. pubens, N. fulva or any of its eight subspecies, or even as an undescribed species. Therefore, at this time it is being identified as N. sp. near pubens (Meyers and Gold 2008), but it could also be referred to as N. sp. -

The Abundance and Mechanical Control of Icerya Purchasi (Maskell) (Hemiptera: Monophlebidae) on Mangifera Indica in Dhaka, Bangladesh
Bangladesh J. Zool. 47(1): 89-96, 2019 ISSN: 0304-9027 (print) 2408-8455 (online) THE ABUNDANCE AND MECHANICAL CONTROL OF ICERYA PURCHASI (MASKELL) (HEMIPTERA: MONOPHLEBIDAE) ON MANGIFERA INDICA IN DHAKA, BANGLADESH Samiha Nowrin, Murshida Begum*, Mousumi Khatun and Moksed Ali Howlader Department of Zoology, University of Dhaka, Dhaka-1000, Bangladesh Abstract: The cottony cushion scale, Icerya purchasi, one of the devastating pests of citrus and ornamentals distributed all over the world. A study was conducted on the biology, abundance and mechanical control of this pest on mango plants from at two locations of Dhaka, Bangladesh. Simple linear regression lines were produced on the lengths and widths of different nymphal instars and adult of this pest. It was proved that body lengths and widths were highly correlated with the successive changing of the nymphal instars from 1st, 2nd and 3rd to adults. The maximum abundance of the I. purchasi on mango leaves was 310 ± 21 in March, 2016. The results of the mechanical control method by hand crushing showed that it was highly effective to control this insect. Abundances of this insect before and after treatment were significantly different (p < 0.05). Abundances of insects in different sampling times were showed different by Tukey’s HSD test (p < 0.05). Key words: Icerya purchasi, Mangifera indica, abundance, mechanical control INTRODUCTION The cottony cushion scale, Icerya purchasi, distributed widely throughout the world and attacks a variety of host plants which has great economic importance (Hale 1970). This is cosmopolitan, abundant in the tropical and subtropical regions in the world. Being euryphagous, their feeding was dependent on a large variety of plants viz., Citrus spp. -

Acacia Flat Mite (Brevipalpus Acadiae Ryke & Meyer, Tenuipalpidae, Acarina): Doringboomplatmyt
Creepie-crawlies and such comprising: Common Names of Insects 1963, indicated as CNI Butterfly List 1959, indicated as BL Some names the sources of which are unknown, and indicated as such Gewone Insekname SKOENLAPPERLYS INSLUITENDE BOSLUISE, MYTE, SAAMGESTEL DEUR DIE AALWURMS EN SPINNEKOPPE LANDBOUTAALKOMITEE Saamgestel deur die MET MEDEWERKING VAN NAVORSINGSINSTITUUT VIR DIE PLANTBESKERMING TAALDIENSBURO Departement van Landbou-tegniese Dienste VAN DIE met medewerking van die DEPARTEMENT VAN ONDERWYS, KUNS EN LANDBOUTAALKOMITEE WETENSKAP van die Taaldiensburo 1959 1963 BUTTERFLY LIST Common Names of Insects COMPILED BY THE INCLUDING TICKS, MITES, EELWORMS AGRICULTURAL TERMINOLOGY AND SPIDERS COMMITTEE Compiled by the IN COLLABORATION WiTH PLANT PROTECTION RESEARCH THE INSTITUTE LANGUAGE SERVICES BUREAU Department of Agricultural Technical Services OF THE in collaboration with the DEPARTMENT OF EDUCATION, ARTS AND AGRICULTURAL TERMINOLOGY SCIENCE COMMITTEE DIE STAATSDRUKKER + PRETORIA + THE of the Language Service Bureau GOVERNMENT PRINTER 1963 1959 Rekenaarmatig en leksikografies herverwerk deur PJ Taljaard e-mail enquiries: [email protected] EXPLANATORY NOTES 1 The list was alphabetised electronically. 2 On the target-language side, ie to the right of the :, synonyms are separated by a comma, e.g.: fission: klowing, splyting The sequence of the translated terms does NOT indicate any preference. Preferred terms are underlined. 3 Where catchwords of similar form are used as different parts of speech and confusion may therefore -

First Record of the Cottony Cushion Scale Icerya Purchasi (Hemiptera, Monophlebidae) in Slovakia – Short Communication
Plant Protect. Sci. Vol. 52, 2016, No. 3: 217–219 doi: 10.17221/23/2016-PPS First Record of the Cottony Cushion Scale Icerya purchasi (Hemiptera, Monophlebidae) in Slovakia – Short Communication Ján KOLLÁR 1, Ladislav BAKAY 1 and Michal PÁSTOR 2 1Horticulture and Landscape Engineering Faculty, Slovak University of Agriculture in Nitra, Nitra, Slovakia; 2Faculty of Ecology and Environmental Sciences, Technical University in Zvolen, Zvolen, Slovak Republic Abstract Kollár J., Bakay L., Pástor M. (2016): First record of the cottony cushion scale Icerya purchasi (Hemiptera, Monophlebidae) in Slovakia – short communication. Plant Protect. Sci., 52: 217–219. Damage by the cottony cushion scale Icerya purchasi (Hemiptera: Coccoidea: Monophlebidae: Iceryini) was found on Rosmarinus officinalis at the locality Suchohrad in Slovakia. Icerya purchasi is a cosmopolitan plant pest of warmer climates. In Central Europe it is a pest of glasshouses. It is the first observation of the cottony cushion scale (at least short-term) occurrence in the outdoor conditions in Slovakia. Keywords: Icerya purchasi; Coccoidea; insect pest; Monophlebidae The cottony cushion scale, Icerya purchasi Maskell, from where it can escape outdoors and survive in 1878 (Hemiptera: Coccoidea: Monophlebidae: Iceryini) favourable conditions even in colder climates. is a cosmopolitan plant pest native to Australia and The cottony cushion scale can be distinguished possibly New Zealand, known on over 200 differ- easily from other scale insects. The mature females ent plant species (Caltagirone & Doutt 1989; (actually parthenogenetic) have bright orange-red, Causton 2001). It has been introduced into other yellow, or brown bodies (Ebeling 1959). The body is parts of the world through global trade. -
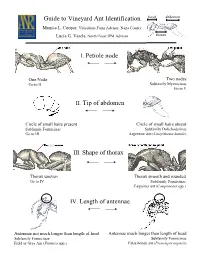
I. Petiole Node II. Tip of Abdomen IV. Length of Antennae Guide to Vineyard Ant Identification III. Shape of Thorax
Guide to Vineyard Ant Identification head abdomen Monica L. Cooper, Viticulture Farm Advisor, Napa County Lucia G. Varela, North Coast IPM Advisor thorax I. Petiole node One Node Two nodes Go to II Subfamily Myrmicinae Go to V II. Tip of abdomen Circle of small hairs present Circle of small hairs absent Subfamily Formicinae Subfamily Dolichoderinae Go to III Argentine Ant (Linepithema humile) III. Shape of thorax Thorax uneven Thorax smooth and rounded Go to IV Subfamily Formicinae Carpenter ant (Camponotus spp.) IV. Length of antennae Antennae not much longer than length of head Antennae much longer than length of head Subfamily Formicinae Subfamily Formicinae Field or Gray Ant (Formica spp.) False honey ant (Prenolepis imparis) head abdomen Petiole with two nodes Subfamily Myrmicinae (V-VIII) thorax V. Dorsal side of Thorax & Antennae One pair of spines on thorax No spines on thorax 12 segmented antennae 10 segmented antennae Go to VI Solenopsis molesta and Solenopsis xyloni VI. Underside of head No brush of bristles Brush of long bristles Go to VII Harvester ants (Pogonomyrmex californicus and P. brevispinosis) VII. Head and Thorax With hairs Without hairs Go to VIII Cardiocondyla mauritanica VIII. Head and Thorax With many parallel furrows Without parallel furrows Profile of thorax rounded Profile of thorax not evenly rounded Pavement ant (Tetramorium “species E”) Pheidole californica Argentine Ant (Linepithema humile), subfamily Dolichoderinae Exotic species 3-4 mm in length Deep brown to light black Move rapidly in distinct trails Feed on honeydew Shallow nests (2 inches from soil surface) Alex Wild Does not bite or sting Carpenter Ant (Camponotus spp.), subfamily Formicinae Large ant: >6 mm in length Dark color with smooth, rounded thorax Workers most active at dusk and night One of most abundant and widespread genera worldwide Generalist scavengers and predators: feed on dead and living insects, nectar, fruit juices and Jack K. -

Pollination Biology of Ailanthus Altissima (Mill.) Swingle (Tree-Of-Heaven) in the Mid- Atlantic United States
Pollination Biology of Ailanthus altissima (Mill.) Swingle (Tree-of-Heaven) in the Mid- Atlantic United States Jessica S. Thompson Thesis submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Master of Science in Life Sciences In Entomology Richard D. Fell Carlyle C. Brewster P. Lloyd Hipkins R. Jay Stipes May 6, 2008 Blacksburg, Virginia Keywords: pollination, Ailanthus altissima, tree-of-heaven, nectar Copyright 2008 Pollination Biology of Ailanthus altissima (Mill.) Swingle (Tree-of-Heaven) in the Mid- Atlantic United States Jessica S. Thompson ABSTRACT To date little information has been collected on the pollination biology of Ailanthus altissima (Mill.) Swingle (tree-of-heaven), an invasive exotic in the U.S. This study was conducted to determine the insect pollinator fauna visiting A. altissima and to study general pollinator visitation patterns associated with the tree’s nectar profile. A list of taxa visiting trees within each of three sites was developed from collected insects. Overall, visitor assemblage was dominated by the soldier beetle Chauliognathus marginatus with large numbers of ants in the genera Formica, Prenolepis, and Camponotus. No major diurnal pattern was found for visitation of insect pollinators using instantaneous counts. The nectar composition, concentration, and amount of total sugars in the flowers of A. altissima and how these are related to tree gender and time of day were determined. Nectar was found to be sucrose-dominant with lower, but nearly equal amounts of fructose and glucose. Total amounts of sugar in male and female blossoms were not statistically different, however higher concentrations of sugar were found in males (40.7%) than in females (35.3%). -

WORLD LIST of EDIBLE INSECTS 2015 (Yde Jongema) WAGENINGEN UNIVERSITY PAGE 1
WORLD LIST OF EDIBLE INSECTS 2015 (Yde Jongema) WAGENINGEN UNIVERSITY PAGE 1 Genus Species Family Order Common names Faunar Distribution & References Remarks life Epeira syn nigra Vinson Nephilidae Araneae Afregion Madagascar (Decary, 1937) Nephilia inaurata stages (Walck.) Nephila inaurata (Walckenaer) Nephilidae Araneae Afr Madagascar (Decary, 1937) Epeira nigra Vinson syn Nephila madagscariensis Vinson Nephilidae Araneae Afr Madagascar (Decary, 1937) Araneae gen. Araneae Afr South Africa Gambia (Bodenheimer 1951) Bostrichidae gen. Bostrichidae Col Afr Congo (DeFoliart 2002) larva Chrysobothris fatalis Harold Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) larva Lampetis wellmani (Kerremans) Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) syn Psiloptera larva wellmani Lampetis sp. Buprestidae Col jewel beetle Afr Togo (Tchibozo 2015) as Psiloptera in Tchibozo but this is Neotropical Psiloptera syn wellmani Kerremans Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) Psiloptera is larva Neotropicalsee Lampetis wellmani (Kerremans) Steraspis amplipennis (Fahr.) Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) larva Sternocera castanea (Olivier) Buprestidae Col jewel beetle Afr Benin (Riggi et al 2013) Burkina Faso (Tchinbozo 2015) Sternocera feldspathica White Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) adult Sternocera funebris Boheman syn Buprestidae Col jewel beetle Afr Zimbabwe (Chavanduka, 1976; Gelfand, 1971) see S. orissa adult Sternocera interrupta (Olivier) Buprestidae Col jewel beetle Afr Benin (Riggi et al 2013) Cameroun (Seignobos et al., 1996) Burkina Faso (Tchimbozo 2015) Sternocera orissa Buquet Buprestidae Col jewel beetle Afr Botswana (Nonaka, 1996), South Africa (Bodenheimer, 1951; syn S. funebris adult Quin, 1959), Zimbabwe (Chavanduka, 1976; Gelfand, 1971; Dube et al 2013) Scarites sp. Carabidae Col ground beetle Afr Angola (Bergier, 1941), Madagascar (Decary, 1937) larva Acanthophorus confinis Laporte de Cast.