Chemical Kinetics (Note -2) the Arrhenius Equation: the Arrhenius
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patnaik-Goldfarb-2016.Pdf
CONTINUOUS ACTIVATION ENERGY REPRESENTATION OF THE ARRHENIUS EQUATION FOR THE PYROLYSIS OF CELLULOSIC MATERIALS: FEED CORN STOVER AND COCOA SHELL BIOMASS * *,** ABHISHEK S. PATNAIK and JILLIAN L. GOLDFARB *Division of Materials Science and Engineering, Boston University, 15 St. Mary’s St., Brookline, MA 02446 **Department of Mechanical Engineering, Boston University, 110 Cummington Mall, Boston, MA 02215 ✉Corresponding author: Jillian L. Goldfarb, [email protected] Received January 22, 2015 Kinetics of lignocellulosic biomass pyrolysis – a pathway for conversion to renewable fuels/chemicals – is transient; discreet changes in reaction rate occur as biomass composition changes over time. There are regimes where activation energy computed via first order Arrhenius function yields a negative value due to a decreasing mass loss rate; this behavior is often neglected in the literature where analyses focus solely on the positive regimes. To probe this behavior feed corn stover and cocoa shells were pyrolyzed at 10 K/min. The activation energies calculated for regimes with positive apparent activation energy for feed corn stover were between 15.3 to 63.2 kJ/mol and for cocoa shell from 39.9 to 89.4 kJ/mol. The regimes with a positive slope (a “negative” activation energy) correlate with evolved concentration of CH4 and C2H2. Given the endothermic nature of pyrolysis, the process is not spontaneous, but the “negative” activation energies represent a decreased devolatilization rate corresponding to the transport of gases from the sample surface. Keywords: Arrhenius equation, biomass pyrolysis, evolved compounds, activation energy INTRODUCTION Fossil fuels comprise the majority of the total energy supply in the world today.1 One of the most critical areas to shift our dependence from fossil to renewable fuels is in energy for transportation, which accounts for well over half of the oil consumed in the United States. -

Eyring Equation
Search Buy/Sell Used Reactors Glass microreactors Hydrogenation Reactor Buy Or Sell Used Reactors Here. Save Time Microreactors made of glass and lab High performance reactor technology Safe And Money Through IPPE! systems for chemical synthesis scale-up. Worldwide supply www.IPPE.com www.mikroglas.com www.biazzi.com Reactors & Calorimeters Induction Heating Reacting Flow Simulation Steam Calculator For Process R&D Laboratories Check Out Induction Heating Software & Mechanisms for Excel steam table add-in for water Automated & Manual Solutions From A Trusted Source. Chemical, Combustion & Materials and steam properties www.helgroup.com myewoss.biz Processes www.chemgoodies.com www.reactiondesign.com Eyring Equation Peter Keusch, University of Regensburg German version "If the Lord Almighty had consulted me before embarking upon the Creation, I should have recommended something simpler." Alphonso X, the Wise of Spain (1223-1284) "Everything should be made as simple as possible, but not simpler." Albert Einstein Both the Arrhenius and the Eyring equation describe the temperature dependence of reaction rate. Strictly speaking, the Arrhenius equation can be applied only to the kinetics of gas reactions. The Eyring equation is also used in the study of solution reactions and mixed phase reactions - all places where the simple collision model is not very helpful. The Arrhenius equation is founded on the empirical observation that rates of reactions increase with temperature. The Eyring equation is a theoretical construct, based on transition state model. The bimolecular reaction is considered by 'transition state theory'. According to the transition state model, the reactants are getting over into an unsteady intermediate state on the reaction pathway. -

Temperature Dependence of Reaction Rates
Module 6 : Reaction Kinetics and Dynamics Lecture 29 : Temperature Dependence of Reaction Rates Objectives In this Lecture you will learn to do the following Give examples of temperature dependence of reaction rate constants (k). Define the activated complex. Outline Arrhenius theory of temperature dependence of k. Rationalize the temperature dependence of molecular speeds and molecular energies through appropriate distribution functions. Summarize other theories for temperature dependence of k. 29.1 Introduction Temperature dependence of physical and chemical parameters is of great interests to chemists and predicting correct temperature dependence is a test as well as a challenge for framing suitable theories. In thermodynamics, temperature dependence of heat capacities and enthalpies was used in calculating equilibrium constants at different temperatures. The departure of real gas behaviour from the ideal gas behaviour is expressed through the temperature dependent viral coefficients. In chemical kinetics, it was observed that for many reactions, increasing the temperature by 10oC, doubled the rates. In rate processes, the temperature dependence is quite striking. In this lecture, we will consider preliminary attempts at explaining this temperature dependence and take up the detailed explanations in later lectures. A knowledge of the distribution of molecular speeds at a given temperature and the population of energy levels is essential in understanding the temperature dependent rate processes and these aspects will also be outlined here. The most common analysis of temperature dependence of reaction rates over a small temperature range of a few tens of degrees Celsius has been through the Arrhenius equation given below. k = A e - Ea / RT (29.1) Where A is the pre exponential factor (commonly referred to as the frequency factor) and Ea is the energy of activation. -

Estimation of Viscosity Arrhenius Pre-Exponential Factor and Activation Energy of Some Organic Liquids E
ISSN 2350-1030 International Journal of Recent Research in Physics and Chemical Sciences (IJRRPCS) Vol. 5, Issue 1, pp: (18-26), Month: April 2018 – September 2018, Available at: www.paperpublications.org ESTIMATION OF VISCOSITY ARRHENIUS PRE-EXPONENTIAL FACTOR AND ACTIVATION ENERGY OF SOME ORGANIC LIQUIDS E. IKE1 and S. C. EZIKE2 1,2Department of Physics, Modibbo Adama University of Technology, P. M. B. 2076, Yola, Adamawa State, Nigeria. Corresponding Author‟s E-mail: [email protected] Abstract: Information concerning fluid’s physico-chemical behaviors is of utmost importance in the design, running and optimization of industrial processes, in this regard, the idea of fluid viscosity quickly comes to mind. Models have been proposed in literatures to describe the viscosity of liquids and fluids in general. The Arrhenius type equations have been proposed for pure solvents; correlating the pre-exponential factor A and activation energy Ea. In this paper, we aim at extending these Arrhenius parameters to simple organic liquids such as water, ethanol and Diethyl ether. Hence, statistical method and analysis are applied using viscosity data set from the literature of some organic liquids. The Arrhenius type equation simplifies the estimation of viscous properties and the calculations thereafter. Keywords: Viscosity, organic liquids, Arrhenius parameters, correlation, statistics. 1. INTRODUCTION All fluids are compressible and when flowing are capable of sustaining shearing stress on account of friction between the adjacent layers. Viscosity (η) is the inherent property of all fluids and may be referred to as the internal friction offered by a fluid to the flow. For water in a beaker, when stirred and left to itself, the motion subsides after sometime, which can happen only in the presence of resisting force acting on the fluid. -

Activation Energy (Ea)
Activation Energy (Ea) Ea value indicates that how temp. changes during processing or storage affect the k value of the reaction. The higher Ea value of the reaction, the more sensitive for the reaction to temp. changes during storage or processing. Ea value is specific for each chemical, microbial and enzymatic reaction. 1 √ Ea cannot be directly measured. √ Ea is calculated from Arrhenius equation. This equation (described by Svante Arrhenius in 1889) gives the relationship between k and temp. of processing or storage. Therefore, we need k and temp. values to determine Ea 2 Arrhenious equation –Ea/ RT k = ko e k: Reaction rate constant (for any reaction order) ko: frequency factor (same unit as k) Ea: Activation energy of the reaction (cal/mole of J/mole) R: Gas constant (1.987 cal/(mole K) or 8.314 J/(mole K) T: Temperature (K) 3 Take ln of both sides – Ea 1 ln k = (—— ——) + ln ko R T ↕ ↕ ↕ ↕ y = a x + b Find the equivalence of this equ. on log10 4 To determine Ea value graphically √ First identify the quality factor of concern and then determine k values at least at three different temp., preferably at five different processing or storage temp. √ Then, plot k values vs 1/T values. Using aritmetic graph paper: Take ln of k values and reciprocal of temp. values in Kelvin and then plot ln k vs 1/T. Slope will be equal to –Ea/R. Using semi-log graph paper: Plot original k values vs 1/T values. Slope will be equal to –Ea/2.303R. -

Eyring Activation Energy Analysis of Acetic Anhydride Hydrolysis in Acetonitrile Cosolvent Systems Nathan Mitchell East Tennessee State University
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 5-2018 Eyring Activation Energy Analysis of Acetic Anhydride Hydrolysis in Acetonitrile Cosolvent Systems Nathan Mitchell East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Analytical Chemistry Commons Recommended Citation Mitchell, Nathan, "Eyring Activation Energy Analysis of Acetic Anhydride Hydrolysis in Acetonitrile Cosolvent Systems" (2018). Electronic Theses and Dissertations. Paper 3430. https://dc.etsu.edu/etd/3430 This Thesis - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. Eyring Activation Energy Analysis of Acetic Anhydride Hydrolysis in Acetonitrile Cosolvent Systems ________________________ A thesis presented to the faculty of the Department of Chemistry East Tennessee State University In partial fulfillment of the requirements for the degree Master of Science in Chemistry ______________________ by Nathan Mitchell May 2018 _____________________ Dr. Dane Scott, Chair Dr. Greg Bishop Dr. Marina Roginskaya Keywords: Thermodynamic Analysis, Hydrolysis, Linear Solvent Energy Relationships, Cosolvent Systems, Acetonitrile ABSTRACT Eyring Activation Energy Analysis of Acetic Anhydride Hydrolysis in Acetonitrile Cosolvent Systems by Nathan Mitchell Acetic anhydride hydrolysis in water is considered a standard reaction for investigating activation energy parameters using cosolvents. Hydrolysis in water/acetonitrile cosolvent is monitored by measuring pH vs. time at temperatures from 15.0 to 40.0 °C and mole fraction of water from 1 to 0.750. -

Reaction Rates: Chemical Kinetics
Chemical Kinetics Reaction Rates: Reaction Rate: The change in the concentration of a reactant or a product with time (M/s). Reactant → Products A → B change in number of moles of B Average rate = change in time ∆()moles of B ∆[B] = = ∆t ∆t ∆[A] Since reactants go away with time: Rate=− ∆t 1 Consider the decomposition of N2O5 to give NO2 and O2: 2N2O5(g)→ 4NO2(g) + O2(g) reactants products decrease with increase with time time 2 From the graph looking at t = 300 to 400 s 0.0009M −61− Rate O2 ==× 9 10 Ms Why do they differ? 100s 0.0037M Rate NO ==× 3.7 10−51 Ms− Recall: 2 100s 0.0019M −51− 2N O (g)→ 4NO (g) + O (g) Rate N O ==× 1.9 10 Ms 2 5 2 2 25 100s To compare the rates one must account for the stoichiometry. 1 Rate O =×× 9 10−−61 Ms =× 9 10 −− 61 Ms 2 1 1 −51−−− 61 Rate NO2 =×× 3.7 10 Ms =× 9.2 10 Ms Now they 4 1 agree! Rate N O =×× 1.9 10−51 Ms−−− = 9.5 × 10 61Ms 25 2 Reaction Rate and Stoichiometry In general for the reaction: aA + bB → cC + dD 11∆∆∆∆[AB] [ ] 11[CD] [ ] Rate ====− − ab∆∆∆ttcdtt∆ 3 Rate Law & Reaction Order The reaction rate law expression relates the rate of a reaction to the concentrations of the reactants. Each concentration is expressed with an order (exponent). The rate constant converts the concentration expression into the correct units of rate (Ms−1). (It also has deeper significance, which will be discussed later) For the general reaction: aA+ bB → cC+ dD x and y are the reactant orders determined from experiment. -

Time-Temperature Superposition WHICH VARIABLES ARE TEMPERATURE DEPENDENT in the ROUSE and REPTATION MODELS?
Time-Temperature Superposition WHICH VARIABLES ARE TEMPERATURE DEPENDENT IN THE ROUSE AND REPTATION MODELS? Rouse Relaxation Modulus: N ρ(T )RT X 2 G(t) = e−tp /λR M p=1 Rouse Relaxation Time: a2N 2ζ(T ) λ = R 6π2kT Reptation Relaxation Modulus: N 8 ρ(T )RT 1 2 X −p t/λd G(t) = 2 2 e π Me p p odd Reptation Relaxation Time: a2N 2ζ(T ) M λd = 2 π kT Me Both models have the same temperature dependence. This is hardly surprising, since reptation is just Rouse motion confined in a tube. Modulus Scale G ∼ ρ(T )T ζ(T ) Time Scale λ ∼ ∼ λ T N Since the friction factor is related to the shortest Rouse mode λN . a2ζ(T ) λ = N 6π2kT 1 Time-Temperature Superposition METHOD OF REDUCED VARIABLES All relaxation modes scale in the same way with temperature. λi(T ) = aT λi(T0) (2-116) aT is a time scale shift factor T0 is a reference temperature (aT ≡ 1 at T = T0). Gi(T0)T ρ Gi(T ) = (2-117) T0ρ0 Recall the Generalized Maxwell Model N X G(t) = Gi exp(−t/λi) (2-25) i=1 with full temperature dependences of the Rouse (and Reptation) Model N T ρ X G(t, T ) = G (T ) exp{−t/[λ (T )a ]} (2-118) T ρ i 0 i 0 T 0 0 i=1 We simplify this by defining reduced variables that are independent of temperature. T ρ G (t) ≡ G(t, T ) 0 0 (2-119) r T ρ t tr ≡ (2-120) aT N X Gr(tr) = Gi(T0) exp[−tr/λi(T0)] (2-118) i=1 Plot of Gr as a function of tr is a universal curve independent of tem- perature. -

A First Course on Kinetics and Reaction Engineering Example 4.6
A First Course on Kinetics and Reaction Engineering Example 4.6 Problem Purpose This example illustrates the use of an Arrhenius plot to determine the best values of a pre- exponential factor and an activation energy. Problem Statement Kinetic studies were performed at a number of different temperatures to find the value of a second order rate coefficient at each of the temperatures. The results are given in the table below. Determine whether the temperature dependence of this rate coefficient is consistent with the Arrhenius equation, and find the best values for the pre-exponential factor and the activation energy. Temperature Rate Coefficient (°C) (L mol-1 min-1) 10 2.63 x 10-4 22 4.78 x 10-4 40 1.52 x 10-3 54 4.18 x 10-3 65 9.07 x 10-3 78 2.14 x 10-2 89 4.2 x 10-2 103 9.42 x 10-2 Problem Analysis This problem clearly involves the use of the Arrhenius expression for the temperature dependence of a rate coefficient. The Arrhenius expression contains two unknown constants, the pre-exponential factor and the activation energy. Here we are given the value of the rate coefficient at eight different temperatures. If we substituted each of those eight data points into the Arrhenius expression, we would have eight equations in two unknowns. Unless the data were “made up” using the Arrhenius expression, there won’t be a single value of the pre-exponential factor and a single value of the activation energy that together satisfy the eight equations. -

Enthalpy and Free Energy of Reaction
KINETICS AND THERMODYNAMICS OF REACTIONS Key words: Enthalpy, entropy, Gibbs free energy, heat of reaction, energy of activation, rate of reaction, rate law, energy profiles, order and molecularity, catalysts INTRODUCTION This module offers a very preliminary detail of basic thermodynamics. Emphasis is given on the commonly used terms such as free energy of a reaction, rate of a reaction, multi-step reactions, intermediates, transition states etc., FIRST LAW OF THERMODYNAMICS. o Law of conservation of energy. States that, • Energy can be neither created nor destroyed. • Total energy of the universe remains constant. • Energy can be converted from one form to another form. eg. Combustion of octane (petrol). 2 C8H18(l) + 25 O2(g) CO2(g) + 18 H2O(g) Conversion of potential energy into thermal energy. → 16 + 10.86 KJ/mol . ESSENTIAL FACTORS FOR REACTION. For a reaction to progress. • The equilibrium must favor the products- • Thermodynamics(energy difference between reactant and product) should be favorable • Reaction rate must be fast enough to notice product formation in a reasonable period. • Kinetics( rate of reaction) ESSENTIAL TERMS OF THERMODYNAMICS. Thermodynamics. Predicts whether the reaction is thermally favorable. The energy difference between the final products and reactants are taken as the guiding principle. The equilibrium will be in favor of products when the product energy is lower. Molecule with lowered energy posses enhanced stability. Essential terms o Free energy change (∆G) – Overall free energy difference between the reactant and the product o Enthalpy (∆H) – Heat content of a system under a given pressure. o Entropy (∆S) – The energy of disorderness, not available for work in a thermodynamic process of a system. -
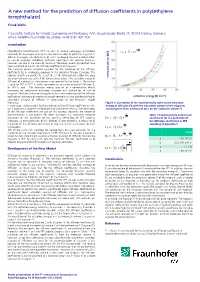
A New Method for the Prediction of Diffusion Coefficients in Poly(Ethylene Terephthalate)
A new method for the prediction of diffusion coefficients in poly(ethylene terephthalate) Frank Welle Fraunhofer Institute for Process Engineering and Packaging (IVV), Giggenhauser Straße 35, 85354 Freising, Germany, email: [email protected], phone: ++49 8161 491 724 Introduction E d A R Poly(ethylene terephthalate) (PET) is used in several packaging applications ] 3 Eq. 4: V c e especially for beverages. Due to the low concentration of potential migrants in food or beverages, the compliance of a PET packaging material is shown often by use of migration modelling. Diffusion coefficients for possible migrants, however, are rare in the scientific literature. Therefore, several approaches have been published to predict the diffusion coefficients of migrants. The currently general accepted equation for the prediction of the diffusion coefficients DP in packaging polymers is the so-called Piringer Equation. The polymer specific parameter AP' is set to 3.1 for temperatures below the glass transition temperature and 6.4 for temperatures above. The activation energy of [Å molecular volume diffusion of a migrant in the polymer is represented by the factor . The current value for PET is 1577 K, which represents an activation energy of diffusion EA of 100 kJ mol-1. This activation energy was set as a conservative default parameter for compliance evaluation purposes and applied for all kind of migrants. This fixed activation energy leads to an over-estimation of the diffusion coefficients, because high molecular weight compounds have significantly higher activation energy [kJ mol-1] activations energies of diffusion in comparison to low molecular weight molecules. Figure 1: Correlation of the experimentally determined activation In conclusion, the equations for the prediction of the diffusion coefficients in PET energy of diffusion EA with the calculated volume of the migrants, (or in polymers in general) are developed for compliance testing. -

Reaction Rates and Temperature; Arrhenius Theory Arrhenius Theory
Reaction Rates and Temperature; Arrhenius Theory CHEM 102 T. Hughbanks Arrhenius Theory −Ea Ae RT k = k is the rate constant! T is the temperature in K! E is the activation energy! R is the ideal-gas constant a (8.314 J/Kmol)! In addition to carrying the units of the rate constant, “A” relates to the frequency of collisions and the orientation of a favorable collision probability! Both A and Ea are specific to a given reaction. ! In order for the reaction to proceed, the reactants ! must posses enough energy to surmount a Eact! reaction barrier. ! "HRXN! PotentialEnergy Reaction Progress! Energy profile for a reaction “activated complex” Rate-determining Ea Quantity reactants Energy ∆E rxn products Thermodynamic Quantity “Reaction Coordinate” The reverse direction... “activated complex” Rate-determining for reverse reaction E (reverse) “products” a Energy ∆E rxn “reactants” Thermodynamic Quantity “Reaction Coordinate” Ea, The Activation Energy Energy of activation for forward reaction:" Ea = Etransition state - Ereactants A reaction can’t proceed unless reactants possess enough energy to give Ea. ∆E, the thermodynamic quantity, tells us about the net reaction. The activation energy, Ea , must be available in the surroundings for the reaction to proceed at a measurable rate. The temperature for a system of particles is described by a distribution of energies. At higher temps, more particles have enough energy to go over the barrier. E > Ea! Since the probability of a E < Ea! molecule reacting increases, the rate increases. The orientation of a molecule during collision can have a profound effect on whether or not a reaction occurs.