Science of Temperature Dependence Freethink Technologies Inc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Eyring Equation
Search Buy/Sell Used Reactors Glass microreactors Hydrogenation Reactor Buy Or Sell Used Reactors Here. Save Time Microreactors made of glass and lab High performance reactor technology Safe And Money Through IPPE! systems for chemical synthesis scale-up. Worldwide supply www.IPPE.com www.mikroglas.com www.biazzi.com Reactors & Calorimeters Induction Heating Reacting Flow Simulation Steam Calculator For Process R&D Laboratories Check Out Induction Heating Software & Mechanisms for Excel steam table add-in for water Automated & Manual Solutions From A Trusted Source. Chemical, Combustion & Materials and steam properties www.helgroup.com myewoss.biz Processes www.chemgoodies.com www.reactiondesign.com Eyring Equation Peter Keusch, University of Regensburg German version "If the Lord Almighty had consulted me before embarking upon the Creation, I should have recommended something simpler." Alphonso X, the Wise of Spain (1223-1284) "Everything should be made as simple as possible, but not simpler." Albert Einstein Both the Arrhenius and the Eyring equation describe the temperature dependence of reaction rate. Strictly speaking, the Arrhenius equation can be applied only to the kinetics of gas reactions. The Eyring equation is also used in the study of solution reactions and mixed phase reactions - all places where the simple collision model is not very helpful. The Arrhenius equation is founded on the empirical observation that rates of reactions increase with temperature. The Eyring equation is a theoretical construct, based on transition state model. The bimolecular reaction is considered by 'transition state theory'. According to the transition state model, the reactants are getting over into an unsteady intermediate state on the reaction pathway. -

Estimation of Viscosity Arrhenius Pre-Exponential Factor and Activation Energy of Some Organic Liquids E
ISSN 2350-1030 International Journal of Recent Research in Physics and Chemical Sciences (IJRRPCS) Vol. 5, Issue 1, pp: (18-26), Month: April 2018 – September 2018, Available at: www.paperpublications.org ESTIMATION OF VISCOSITY ARRHENIUS PRE-EXPONENTIAL FACTOR AND ACTIVATION ENERGY OF SOME ORGANIC LIQUIDS E. IKE1 and S. C. EZIKE2 1,2Department of Physics, Modibbo Adama University of Technology, P. M. B. 2076, Yola, Adamawa State, Nigeria. Corresponding Author‟s E-mail: [email protected] Abstract: Information concerning fluid’s physico-chemical behaviors is of utmost importance in the design, running and optimization of industrial processes, in this regard, the idea of fluid viscosity quickly comes to mind. Models have been proposed in literatures to describe the viscosity of liquids and fluids in general. The Arrhenius type equations have been proposed for pure solvents; correlating the pre-exponential factor A and activation energy Ea. In this paper, we aim at extending these Arrhenius parameters to simple organic liquids such as water, ethanol and Diethyl ether. Hence, statistical method and analysis are applied using viscosity data set from the literature of some organic liquids. The Arrhenius type equation simplifies the estimation of viscous properties and the calculations thereafter. Keywords: Viscosity, organic liquids, Arrhenius parameters, correlation, statistics. 1. INTRODUCTION All fluids are compressible and when flowing are capable of sustaining shearing stress on account of friction between the adjacent layers. Viscosity (η) is the inherent property of all fluids and may be referred to as the internal friction offered by a fluid to the flow. For water in a beaker, when stirred and left to itself, the motion subsides after sometime, which can happen only in the presence of resisting force acting on the fluid. -

Eyring Activation Energy Analysis of Acetic Anhydride Hydrolysis in Acetonitrile Cosolvent Systems Nathan Mitchell East Tennessee State University
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 5-2018 Eyring Activation Energy Analysis of Acetic Anhydride Hydrolysis in Acetonitrile Cosolvent Systems Nathan Mitchell East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Analytical Chemistry Commons Recommended Citation Mitchell, Nathan, "Eyring Activation Energy Analysis of Acetic Anhydride Hydrolysis in Acetonitrile Cosolvent Systems" (2018). Electronic Theses and Dissertations. Paper 3430. https://dc.etsu.edu/etd/3430 This Thesis - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. Eyring Activation Energy Analysis of Acetic Anhydride Hydrolysis in Acetonitrile Cosolvent Systems ________________________ A thesis presented to the faculty of the Department of Chemistry East Tennessee State University In partial fulfillment of the requirements for the degree Master of Science in Chemistry ______________________ by Nathan Mitchell May 2018 _____________________ Dr. Dane Scott, Chair Dr. Greg Bishop Dr. Marina Roginskaya Keywords: Thermodynamic Analysis, Hydrolysis, Linear Solvent Energy Relationships, Cosolvent Systems, Acetonitrile ABSTRACT Eyring Activation Energy Analysis of Acetic Anhydride Hydrolysis in Acetonitrile Cosolvent Systems by Nathan Mitchell Acetic anhydride hydrolysis in water is considered a standard reaction for investigating activation energy parameters using cosolvents. Hydrolysis in water/acetonitrile cosolvent is monitored by measuring pH vs. time at temperatures from 15.0 to 40.0 °C and mole fraction of water from 1 to 0.750. -

Reaction Rates: Chemical Kinetics
Chemical Kinetics Reaction Rates: Reaction Rate: The change in the concentration of a reactant or a product with time (M/s). Reactant → Products A → B change in number of moles of B Average rate = change in time ∆()moles of B ∆[B] = = ∆t ∆t ∆[A] Since reactants go away with time: Rate=− ∆t 1 Consider the decomposition of N2O5 to give NO2 and O2: 2N2O5(g)→ 4NO2(g) + O2(g) reactants products decrease with increase with time time 2 From the graph looking at t = 300 to 400 s 0.0009M −61− Rate O2 ==× 9 10 Ms Why do they differ? 100s 0.0037M Rate NO ==× 3.7 10−51 Ms− Recall: 2 100s 0.0019M −51− 2N O (g)→ 4NO (g) + O (g) Rate N O ==× 1.9 10 Ms 2 5 2 2 25 100s To compare the rates one must account for the stoichiometry. 1 Rate O =×× 9 10−−61 Ms =× 9 10 −− 61 Ms 2 1 1 −51−−− 61 Rate NO2 =×× 3.7 10 Ms =× 9.2 10 Ms Now they 4 1 agree! Rate N O =×× 1.9 10−51 Ms−−− = 9.5 × 10 61Ms 25 2 Reaction Rate and Stoichiometry In general for the reaction: aA + bB → cC + dD 11∆∆∆∆[AB] [ ] 11[CD] [ ] Rate ====− − ab∆∆∆ttcdtt∆ 3 Rate Law & Reaction Order The reaction rate law expression relates the rate of a reaction to the concentrations of the reactants. Each concentration is expressed with an order (exponent). The rate constant converts the concentration expression into the correct units of rate (Ms−1). (It also has deeper significance, which will be discussed later) For the general reaction: aA+ bB → cC+ dD x and y are the reactant orders determined from experiment. -

Time-Temperature Superposition WHICH VARIABLES ARE TEMPERATURE DEPENDENT in the ROUSE and REPTATION MODELS?
Time-Temperature Superposition WHICH VARIABLES ARE TEMPERATURE DEPENDENT IN THE ROUSE AND REPTATION MODELS? Rouse Relaxation Modulus: N ρ(T )RT X 2 G(t) = e−tp /λR M p=1 Rouse Relaxation Time: a2N 2ζ(T ) λ = R 6π2kT Reptation Relaxation Modulus: N 8 ρ(T )RT 1 2 X −p t/λd G(t) = 2 2 e π Me p p odd Reptation Relaxation Time: a2N 2ζ(T ) M λd = 2 π kT Me Both models have the same temperature dependence. This is hardly surprising, since reptation is just Rouse motion confined in a tube. Modulus Scale G ∼ ρ(T )T ζ(T ) Time Scale λ ∼ ∼ λ T N Since the friction factor is related to the shortest Rouse mode λN . a2ζ(T ) λ = N 6π2kT 1 Time-Temperature Superposition METHOD OF REDUCED VARIABLES All relaxation modes scale in the same way with temperature. λi(T ) = aT λi(T0) (2-116) aT is a time scale shift factor T0 is a reference temperature (aT ≡ 1 at T = T0). Gi(T0)T ρ Gi(T ) = (2-117) T0ρ0 Recall the Generalized Maxwell Model N X G(t) = Gi exp(−t/λi) (2-25) i=1 with full temperature dependences of the Rouse (and Reptation) Model N T ρ X G(t, T ) = G (T ) exp{−t/[λ (T )a ]} (2-118) T ρ i 0 i 0 T 0 0 i=1 We simplify this by defining reduced variables that are independent of temperature. T ρ G (t) ≡ G(t, T ) 0 0 (2-119) r T ρ t tr ≡ (2-120) aT N X Gr(tr) = Gi(T0) exp[−tr/λi(T0)] (2-118) i=1 Plot of Gr as a function of tr is a universal curve independent of tem- perature. -

A First Course on Kinetics and Reaction Engineering Example 4.6
A First Course on Kinetics and Reaction Engineering Example 4.6 Problem Purpose This example illustrates the use of an Arrhenius plot to determine the best values of a pre- exponential factor and an activation energy. Problem Statement Kinetic studies were performed at a number of different temperatures to find the value of a second order rate coefficient at each of the temperatures. The results are given in the table below. Determine whether the temperature dependence of this rate coefficient is consistent with the Arrhenius equation, and find the best values for the pre-exponential factor and the activation energy. Temperature Rate Coefficient (°C) (L mol-1 min-1) 10 2.63 x 10-4 22 4.78 x 10-4 40 1.52 x 10-3 54 4.18 x 10-3 65 9.07 x 10-3 78 2.14 x 10-2 89 4.2 x 10-2 103 9.42 x 10-2 Problem Analysis This problem clearly involves the use of the Arrhenius expression for the temperature dependence of a rate coefficient. The Arrhenius expression contains two unknown constants, the pre-exponential factor and the activation energy. Here we are given the value of the rate coefficient at eight different temperatures. If we substituted each of those eight data points into the Arrhenius expression, we would have eight equations in two unknowns. Unless the data were “made up” using the Arrhenius expression, there won’t be a single value of the pre-exponential factor and a single value of the activation energy that together satisfy the eight equations. -

Enthalpy and Free Energy of Reaction
KINETICS AND THERMODYNAMICS OF REACTIONS Key words: Enthalpy, entropy, Gibbs free energy, heat of reaction, energy of activation, rate of reaction, rate law, energy profiles, order and molecularity, catalysts INTRODUCTION This module offers a very preliminary detail of basic thermodynamics. Emphasis is given on the commonly used terms such as free energy of a reaction, rate of a reaction, multi-step reactions, intermediates, transition states etc., FIRST LAW OF THERMODYNAMICS. o Law of conservation of energy. States that, • Energy can be neither created nor destroyed. • Total energy of the universe remains constant. • Energy can be converted from one form to another form. eg. Combustion of octane (petrol). 2 C8H18(l) + 25 O2(g) CO2(g) + 18 H2O(g) Conversion of potential energy into thermal energy. → 16 + 10.86 KJ/mol . ESSENTIAL FACTORS FOR REACTION. For a reaction to progress. • The equilibrium must favor the products- • Thermodynamics(energy difference between reactant and product) should be favorable • Reaction rate must be fast enough to notice product formation in a reasonable period. • Kinetics( rate of reaction) ESSENTIAL TERMS OF THERMODYNAMICS. Thermodynamics. Predicts whether the reaction is thermally favorable. The energy difference between the final products and reactants are taken as the guiding principle. The equilibrium will be in favor of products when the product energy is lower. Molecule with lowered energy posses enhanced stability. Essential terms o Free energy change (∆G) – Overall free energy difference between the reactant and the product o Enthalpy (∆H) – Heat content of a system under a given pressure. o Entropy (∆S) – The energy of disorderness, not available for work in a thermodynamic process of a system. -
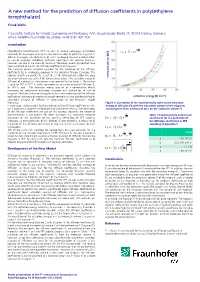
A New Method for the Prediction of Diffusion Coefficients in Poly(Ethylene Terephthalate)
A new method for the prediction of diffusion coefficients in poly(ethylene terephthalate) Frank Welle Fraunhofer Institute for Process Engineering and Packaging (IVV), Giggenhauser Straße 35, 85354 Freising, Germany, email: [email protected], phone: ++49 8161 491 724 Introduction E d A R Poly(ethylene terephthalate) (PET) is used in several packaging applications ] 3 Eq. 4: V c e especially for beverages. Due to the low concentration of potential migrants in food or beverages, the compliance of a PET packaging material is shown often by use of migration modelling. Diffusion coefficients for possible migrants, however, are rare in the scientific literature. Therefore, several approaches have been published to predict the diffusion coefficients of migrants. The currently general accepted equation for the prediction of the diffusion coefficients DP in packaging polymers is the so-called Piringer Equation. The polymer specific parameter AP' is set to 3.1 for temperatures below the glass transition temperature and 6.4 for temperatures above. The activation energy of [Å molecular volume diffusion of a migrant in the polymer is represented by the factor . The current value for PET is 1577 K, which represents an activation energy of diffusion EA of 100 kJ mol-1. This activation energy was set as a conservative default parameter for compliance evaluation purposes and applied for all kind of migrants. This fixed activation energy leads to an over-estimation of the diffusion coefficients, because high molecular weight compounds have significantly higher activation energy [kJ mol-1] activations energies of diffusion in comparison to low molecular weight molecules. Figure 1: Correlation of the experimentally determined activation In conclusion, the equations for the prediction of the diffusion coefficients in PET energy of diffusion EA with the calculated volume of the migrants, (or in polymers in general) are developed for compliance testing. -

Reaction Rates and Temperature; Arrhenius Theory Arrhenius Theory
Reaction Rates and Temperature; Arrhenius Theory CHEM 102 T. Hughbanks Arrhenius Theory −Ea Ae RT k = k is the rate constant! T is the temperature in K! E is the activation energy! R is the ideal-gas constant a (8.314 J/Kmol)! In addition to carrying the units of the rate constant, “A” relates to the frequency of collisions and the orientation of a favorable collision probability! Both A and Ea are specific to a given reaction. ! In order for the reaction to proceed, the reactants ! must posses enough energy to surmount a Eact! reaction barrier. ! "HRXN! PotentialEnergy Reaction Progress! Energy profile for a reaction “activated complex” Rate-determining Ea Quantity reactants Energy ∆E rxn products Thermodynamic Quantity “Reaction Coordinate” The reverse direction... “activated complex” Rate-determining for reverse reaction E (reverse) “products” a Energy ∆E rxn “reactants” Thermodynamic Quantity “Reaction Coordinate” Ea, The Activation Energy Energy of activation for forward reaction:" Ea = Etransition state - Ereactants A reaction can’t proceed unless reactants possess enough energy to give Ea. ∆E, the thermodynamic quantity, tells us about the net reaction. The activation energy, Ea , must be available in the surroundings for the reaction to proceed at a measurable rate. The temperature for a system of particles is described by a distribution of energies. At higher temps, more particles have enough energy to go over the barrier. E > Ea! Since the probability of a E < Ea! molecule reacting increases, the rate increases. The orientation of a molecule during collision can have a profound effect on whether or not a reaction occurs. -

Dynamic NMR Experiments: -Dr
Determining the Energy of Activation Parameters from Dynamic NMR Experiments: -Dr. Rich Shoemaker (Source: Dynamic NMR Spectroscopy by J. Sandstöm, and me) • The results contained in this document have been published: o Zimmer, Shoemaker, & Ruminski, Inorganica Chimica Acta, 359(2006) 1478-1484 The most common (and oft inaccurate) method of determining activation energy parameters is through the determination (often estimation) of temperature at which the NMR resonances of 2 exchanging species coalesce. The coalescence temperature (Tc) is then used in conjunction with the maximum peak separation in the low-temperature (i.e. slow-exchange) limit (∆ν in Hz). The biggest source of error using the method of coalescence is (1) accurately determining Tc and (2) accurately determining ∆ν. Often, the isotropic chemical shifts of the exchanging species are temperature dependent, so ∆ν changes with temperature. If this happens, then the error in estimation of the activation energy barrier (∆G‡) can be very large. k The rate constant (k ) in these calculations, for nearly all NMR exchange 1 r A B situations, is actually k1+k2 in a system for A exchanging with B, where: k2 The equation to estimate ∆G‡ using the coalescence temperature is: ‡ Tc -3 ∆G = aT 9.972 + log where a = 4.575 x 10 for units of kcal/mol ∆ν a = 1.914 x 10-2 for units of kJ/mol Note: at the coalescence temperature, kc = π∆υ/√2 A second common method of determining the energy of activation (Ea) is by performing an Arrhenius Plot. If one knows the exchange rate constant (kr) at several temperatures (always in Kelvin), one can plot ln(k) vs. -

A New Attempt to Better Understand Arrehnius Equation and Its Activation Energy
Chapter 4 A New Attempt to Better Understand Arrehnius Equation and Its Activation Energy Andrzej Kulczycki and Czesław Kajdas Additional information is available at the end of the chapter http://dx.doi.org/10.5772/54503 1. Introduction Activation energy (Ea) is strictly combined with kinetics of chemical reactions. The relationship is described by Arrehnius equation k = Aexp(-Ea/RT) (1) where k is the rate coefficient, A is a constant, R is the universal gas constant, and T is the temperature (in Kelvin); R has the value of 8.314 x 10-3 kJ mol-1K-1, Ea is the amount of energy required to ensure that a reaction happens. Common sense is that at higher temperatures, the probability of two molecules colliding is higher. Accordingly, a given reaction rate is higher and the reaction proceeds faster and, the effect of temperature on reaction rates is calculated using the Arrhenius equation. Further reaction rate enhancement is promoted by catalysis. Catalysis is the phenomenon of a catalyst in action. Catalyst is a material that increases the rate of chemical reaction, and for equilibrium reactions it increases the rate at which a chemical system approaches equilibrium, without being consumed in the process [1]. It is applied in small amounts relative to the reactants. Chemical kinetics, based on Arrhenius equation assumes that catalysts lower the activation energy (Ea) and the presence of catalyst results higher reaction rate at the same temperature. In chemical kinetics Ea is the height of the potential barrier separating the products and reactants. Catalytic reactions have a lower Ea than those of the thermally activated. -

Table of Recommended Rate Constants for Chemical Reactions Occurring in Combustion
A111D2 14bB cn 0- NATL INST OF STANMffii.SSJ All 1021 46399 1964 ffiSWR***""" NBS-PUB-C « JC $ to NSRDS-NBS 67 \ %J 0* U.S. DEPARTMENT OF COMMERCE / National Bureau of Standards U, 2 NATIONAL BUREAU OF STANDARDS The National Bureau of Standards' was established by an act of Congress on March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation’s physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau’s technical work is per- formed by the National Measurement Laboratory, the National Engineering Laboratory, and the Institute for Computer Sciences and Technology. THE NATIONAL MEASUREMENT LABORATORY provides the national system of physical and chemical and materials measurement; coordinates the system with measurement systems of other nations and furnishes essential services leading to accurate and uniform physical and chemical measurement throughout the Nation’s scientific community, industry, and commerce; conducts materials research leading to improved methods of measurement, standards, and data on the properties of materials needed by industry, commerce, educational institutions, and Government; provides advisory and research services to other Government agencies; develops, produces, and distributes Standard Reference Materials; and provides calibration services. The Laboratory consists of the following centers: Absolute Physical Quantities 2 — Radiation Research — Thermodynamics and Molecular Science — Analytical Chemistry — Materials Science.