Perissodactyla: Tapirus) Exposed Using Geometric Morphometrics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Of Vertebrate Fossils from the Middle Eocene Oil Shale of Messel, Germany: Implications for Their Taphonomy and Palaeoenvironment
Palaeogeography, Palaeoclimatology, Palaeoecology 416 (2014) 92–109 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Isotope compositions (C, O, Sr, Nd) of vertebrate fossils from the Middle Eocene oil shale of Messel, Germany: Implications for their taphonomy and palaeoenvironment Thomas Tütken ⁎ Steinmann-Institut für Geologie, Mineralogie und Paläontologie, Universität Bonn, Poppelsdorfer Schloss, 53115 Bonn, Germany article info abstract Article history: The Middle Eocene oil shale deposits of Messel are famous for their exceptionally well-preserved, articulated 47- Received 15 April 2014 Myr-old vertebrate fossils that often still display soft tissue preservation. The isotopic compositions (O, C, Sr, Nd) Received in revised form 30 July 2014 were analysed from skeletal remains of Messel's terrestrial and aquatic vertebrates to determine the condition of Accepted 5 August 2014 geochemical preservation. Authigenic phosphate minerals and siderite were also analysed to characterise the iso- Available online 17 August 2014 tope compositions of diagenetic phases. In Messel, diagenetic end member values of the volcanically-influenced 12 Keywords: and (due to methanogenesis) C-depleted anoxic bottom water of the meromictic Eocene maar lake are isoto- Strontium isotopes pically very distinct from in vivo bioapatite values of terrestrial vertebrates. This unique taphonomic setting al- Oxygen isotopes lows the assessment of the geochemical preservation of the vertebrate fossils. A combined multi-isotope Diagenesis approach demonstrates that enamel of fossil vertebrates from Messel is geochemically exceptionally well- Enamel preserved and still contains near-in vivo C, O, Sr and possibly even Nd isotope compositions while bone and den- Messel tine are diagenetically altered. -

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Perissodactyla: Tapirus) Hints at Subtle Variations in Locomotor Ecology
JOURNAL OF MORPHOLOGY 277:1469–1485 (2016) A Three-Dimensional Morphometric Analysis of Upper Forelimb Morphology in the Enigmatic Tapir (Perissodactyla: Tapirus) Hints at Subtle Variations in Locomotor Ecology Jamie A. MacLaren1* and Sandra Nauwelaerts1,2 1Department of Biology, Universiteit Antwerpen, Building D, Campus Drie Eiken, Universiteitsplein, Wilrijk, Antwerp 2610, Belgium 2Centre for Research and Conservation, Koninklijke Maatschappij Voor Dierkunde (KMDA), Koningin Astridplein 26, Antwerp 2018, Belgium ABSTRACT Forelimb morphology is an indicator for order Perissodactyla (odd-toed ungulates). Modern terrestrial locomotor ecology. The limb morphology of the tapirs are widely accepted to belong to a single enigmatic tapir (Perissodactyla: Tapirus) has often been genus (Tapirus), containing four extant species compared to that of basal perissodactyls, despite the lack (Hulbert, 1973; Ruiz-Garcıa et al., 1985) and sev- of quantitative studies comparing forelimb variation in eral regional subspecies (Padilla and Dowler, 1965; modern tapirs. Here, we present a quantitative assess- ment of tapir upper forelimb osteology using three- Wilson and Reeder, 2005): the Baird’s tapir (T. dimensional geometric morphometrics to test whether bairdii), lowland tapir (T. terrestris), mountain the four modern tapir species are monomorphic in their tapir (T. pinchaque), and the Malayan tapir (T. forelimb skeleton. The shape of the upper forelimb bones indicus). Extant tapirs primarily inhabit tropical across four species (T. indicus; T. bairdii; T. terrestris; T. rainforest, with some populations also occupying pinchaque) was investigated. Bones were laser scanned wet grassland and chaparral biomes (Padilla and to capture surface morphology and 3D landmark analysis Dowler, 1965; Padilla et al., 1996). was used to quantify shape. -

Critical Comments on the Genus Propachynolophus Lemoine, 1891 (Mammalia, Perissodactyla, Equoidea)
ARTICLE Critical comments on the genus Propachynolophus Lemoine, 1891 (Mammalia, Perissodactyla, Equoidea) JEAN A. REMY Institut des Sciences de l’Evolution, UMR 5554 CNRS, IRD, EPHE, Université de Montpellier, Place Eugène Bataillon, 34095 Montpellier cedex 5, France E-mail: [email protected] Abstract: The validity of Propachynolophus Lemoine, 1891, supposedly an intermediate between Hyracotherium Owen, 1841 and Pachynolophus Pomel, 1847, has been questioned for a long time. A detailed analysis of features on which this genus is based further supported by a formal cladistic analysis demonstrates that Propachynolophus is not a valid taxon. The type species, “Propachynolophus gaudryi Lemoine, 1891” shall be assigned to Propalaeotherium Gervais, 1849, under the new combination Propalaeotherium gaudryi (Lemoine, 1891). “Pachynolophus maldani Lemoine, 1878”, later assigned to Propachynolophus, typifies the new genus Orolophus, under the binomen Orolophus maldani (Lemoine, 1878). The other referred species, “Propachynolophus levei Hooker, 1994” and “P. remyi Checa-Soler, 1997” are poorly documented, and both species shall be provisionally referred to as “Hyracotherium” levei (Hooker, 1994) and “Hyracotherium” remyi (Checa-Soler, 1997), pending new discoveries. Keywords: Eocene, tooth morphology, Pachynolophus, Propalaeotherium, Eurohippus Submitted 8 December 2016, Accepted 3 August 2017 Published Online 27 September 2017, doi: 1018563/pv.41.1.e3 © Copyright Jean Remy September 2017 INTRODUCTION Propachynolophus gaudryi was “based upon miscellaneous isolated teeth and jaw fragments constituting a part of the fossil vertebrate sample collected in the vicinity of Epernay (Marne)” By their diversity and abundance, perissodactyls have played (Savage et al., 1965: 16), a sample known as the “Ageian a crucial role in the European Eocene mammalian faunas. -
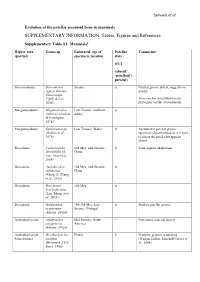
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp. -

Late Miocene Tapirus(Mammalia
Bull. Fla. Mus. Nat. Hist. (2005) 45(4): 465-494 465 LATE MIOCENE TAPIRUS (MAMMALIA, PERISSODACTYLA) FROM FLORIDA, WITH DESCRIPTION OF A NEW SPECIES, TAPIRUS WEBBI Richard C. Hulbert Jr.1 Tapirus webbi n. sp. is a relatively large tapir from north-central Florida with a chronologic range of very late Clarendonian (Cl3) to very early Hemphillian (Hh1), or ca. 9.5 to 7.5 Ma. It is about the size of extant Tapirus indicus but with longer limbs. Tapirus webbi differs from Tapirus johnsoni (Cl3 of Nebraska) by its larger size, relatively shorter diastema, thicker nasal, and better developed transverse lophs on premolars. Tapirus webbi is more similar to Tapirus simpsoni from the late early Hemphillian (Hh2, ca. 7 Ma) of Nebraska, but differs in having narrower upper premolars and weaker transverse lophs on P1 and P2. Tapirus webbi differs from North American Plio-Pleistocene species such as Tapirus veroensis and Tapirus haysii in its polygonal (not triangu- lar) interparietal, spicular posterior lacrimal process, relatively narrow P2-M3, and lack of an extensive meatal diverticulum fossa on the dorsal surface of the nasal. In Florida, Hh2 Tapirus is known only from relatively incomplete specimens, but at least two species are represented, both of significantly smaller size than Tapirus webbi or Tapirus simpsoni. One appears to be the dwarf Tapirus polkensis (Olsen), previously known from the very late Hemphillian (Hh4) in Florida and the Hemphillian of Tennessee (referred specimens from Nebraska need to be reexamined). Previous interpretations that the age of T. polkensis is middle Miocene are incorrect; its chronologic range in Florida is Hh2 to Hh4 based on direct association with biochronologic indicator taxa such as Neohipparion eurystyle, Dinohippus mexicanus and Agriotherium schneideri. -

New Early Eocene Basal Tapiromorph from Southern China and Its Phylogenetic Implications
New Early Eocene Basal tapiromorph from Southern China and Its Phylogenetic Implications Bin Bai1,2*, Yuanqing Wang1*, Jin Meng2,1, Qian Li1, Xun Jin1 1 Key Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China, 2 Division of Paleontology, American Museum of Natural History, New York, New York, United States of America Abstract A new Early Eocene tapiromorph, Meridiolophus expansus gen. et sp. nov., from the Sanshui Basin, Guangdong Province, China, is described and discussed. It is the first reported Eocene mammal from the basin. The new taxon, represented by a left fragmentary mandible, is characterized by an expanded anterior symphyseal region, a long diastema between c1 and p1, a rather short diastema between p1 and p2, smaller premolars relative to molars, an incipient metaconid appressed to the protoconid on p3, a prominent entoconid on p4, molar metaconid not twinned, cristid obliqua extending mesially and slightly lingually from the hypoconid, inclined metalophid and hypolophid, and small hypoconulid on the lower preultimate molars. Meridiolophus is morphologically intermediate between basal Homogalax-like taxa and derived tapiromorphs (such as Heptodon). Phylogenetic analysis indicates Equidae is more closely related to Tapiromorpha than to Palaeotheriidae, although the latter is only represented by a single species Pachynolophus eulaliensis. ‘Isectolophidae’, with exception of Meridiolophus and Karagalax, has the closest affinity with Chalicotherioidea. Furthermore, the majority rule consensus tree shows that Meridiolophus is closer to Karagalax than to any other ‘isectolophid’, and both genera represent stem taxa to crown group Ceratomorpha. Citation: Bai B, Wang Y, Meng J, Li Q, Jin X (2014) New Early Eocene Basal tapiromorph from Southern China and Its Phylogenetic Implications. -

Bit by Bit: an Iconographic Study of Horses in the Reliefs of the Assyrian King Ashurnasirpal II (883-859BC)
Bit by Bit: an Iconographic study of horses in the reliefs of the Assyrian king Ashurnasirpal II (883-859BC) Stephanie Baldwin Thesis presented in partial fulfilment of the requirements for the degree of Master of Philosophy in the Faculty of Arts and Social Sciences at the University of Stellenbosch Supervisor: Professor I. Cornelius April 2014 Stellenbosch University http://scholar.sun.ac.za Declaration By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. December 2013 Copyright © 2014 Stellenbosch University All rights reserved Stellenbosch University http://scholar.sun.ac.za Abstract - English: The focus of this study is to investigate the role that horses played in the Ancient Near East, specifically during the reign of the Neo-Assyrian king Ashurnasirpal II (883-859BC). By studying nine of the reliefs from the North-West Palace at Nimrud, the function of horses with regard to warfare during that time was explored. The analysis included an examination of all horses and equine tack, which consists of chariots, bridles, bits, breastplates and decorations. The reliefs are studied by using Erwin Panofsky’s Theoretical Scheme, which allows for three stages of analysis. Each of the reliefs is examined as a whole, in order to place the relief in context, followed by a detailed breakdown of the horses, specifically their body language, as well as their tack and the function thereof. -

This Item Is the Archived Peer-Reviewed Author-Version Of
This item is the archived peer-reviewed author-version of: Modern tapirs as morphofunctional analogues for locomotion in endemic eocene European perissodactyls Reference: Maclaren Jamie, Nauw elaerts Sandra.- Modern tapirs as morphofunctional analogues for locomotion in endemic eocene European perissodactyls JOURNAL OF MAMMALIAN EVOLUTION - ISSN 1064-7554 - (2019), p. 1-19 Full text (Publisher's DOI): https://doi.org/10.1007/S10914-019-09460-1 To cite this reference: https://hdl.handle.net/10067/1580640151162165141 Institutional repository IRUA 1 TITLE: 2 Modern tapirs as morphofunctional analogues for locomotion 3 in endemic Eocene European perissodactyls 4 Jamie A. MacLaren1* and Sandra Nauwelaerts1,2 5 6 1 Department of Biology, Universiteit Antwerpen, Campus Drie Eiken, Universiteitsplein, Wilrijk, 7 Antwerp, 2610 (Belgium). 8 2 Center for Research and Conservation, Koninklijke Maatschappij voor Dierkunde (KMDA), 9 Koningin Astridplein 26, Antwerp, 2018 (Belgium). 10 11 12 * Corresponding Author 13 14 15 Corresponding Author: Jamie MacLaren, Room D.1.41, Department of Biology, Universiteit 16 Antwerpen, Campus Drie Eiken, Universiteitsplein, Wilrijk, Antwerp, 2610 (Belgium) 17 18 ORCID: 19 JM (0000-0003-4177-227X) 20 SN (0000-0002-2289-4477) 21 Abstract 22 Tapirs have historically been considered as ecologically analogous to several groups of extinct 23 perissodactyls based on dental and locomotor morphology. Here, we investigate comparative 24 functional morphology between living tapirs and endemic Eocene European perissodactyls to 25 ascertain whether tapirs represent viable analogues for locomotion in palaeotheres and lophiodontids. 26 Forelimb bones from 20 species of Eocene European perissodactyls were laser scanned and 27 compared to a forelimb dataset of extant Tapirus. Bone shape was quantified using 3D geometric 28 morphometrics; coordinates were Procrustes aligned and compared using Principal Component 29 Analysis and neighbor-joining trees. -

Smithsonian Miscellaneous Collections
SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 149, NUMBER 2 CljarlesJ ©. anb iWarp "^aux OTalcott 3Res;earcf) jFunb A STUDY OF THE EARLY TERTIARY CONDYLARTHRAN MAMMAL MENISCOTHERIUM (With U Plates) By C. LEWIS GAZIN Curator, Division of Vertebrate Paleontology U. S. National Museum Smithsonian Institution (Publication 4605) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION MAY 10, 1965 LIBRARY OF THE AMBKICAN MUSEUM OF NATURAL HISTORY SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 149, NUMBER 2 Cfjatles; JB. anb JHarp "^aux OTalcott iResicarcf) jFunb A STUDY OF THE EARLY TERTIARY CONDYLARTHRAN MAMMAL MENISCOTHERIUM (With 11 Plates) By C. LEWIS GAZIN Curator, Division of Vertebrate Paleontology U. S. National Museum Smithsonian Institution <s»I^****»5?' (Publication 4605) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION MAY 10, 1965 PORT CITY PRESS, INC. BALTIMORE, MD., U. S. A. CONTENTS Page Introduction 1 Acknowledgments 2 History of investigation 3 Geographic and geologic occurrence 9 Environment 13 Classification 20 The skeleton of Meniscotherium, with notes on Phenacodns, Hyopsodns, and other condylarths 22 Skull 22 Endocranial cast 35 Mandible 39 Dentition 41 Vertebrae 45 Scapula 50 Humerus 51 Radius SZ Ulna 54 Manus 55 Pelvic girdle 64 Femur 65 Tibia 67 Fibula 69 Pes 70 Summary of relationships 81 References 90 Explanation of plates 96 ILLUSTRATIONS PLATES (All plates following p. 98.) 1. Mcniscothcrunn skull from the early Eocene of Wyoming. 2. Meniscotherium skull from the early Eocene of Wyoming. 3. Meniscotherium from the early Eocene of Wyoming. 4. Meniscotherium type specimens. 5. Meniscotherium dentitions from the early Eocene of Wyoming. 6. Meniscotherium humerus and scapula from the early Eocene of Wyoming. -

The Eocene Ge
Anuário do Instituto de Geociências - UFRJ www.anuario.igeo.ufrj.br The Former Geiseltal Museum (1934-2011), the Eocene Geiseltal Fossilagerstätte (Germany) and the Scientific Meaning of Ben Barnes as a Pioneer of Systematic Quantitative Vertebrate Excavations in the Geiseltal Lignites O Antigo Museu Geiseltal (1934-2011), a Fossilagerstätte Eocênica Geiseltal (Alemanha) e o Significado Científico de Ben Barnes como Pioneiro das Escavações Quantitativas Sistemáticas de Vertebrados nos Linhitos Geiseltal Meinolf Hellmund† Martin-Luther-Universität Halle-Wittenberg, Zentralmagazin Naturwissenschaftlicher Sammlungen, Geiseltalsammlung, Domplatz 4, 06108, Halle, Saale, Germany. †in memoriam E-mail: [email protected] Recebido em: 22/02/2017 Aprovado em: 20/02/2018 DOI: http://dx.doi.org/10.11137/2018_1_108_119 Abstract The Geiseltal was a productive area for mining of lignite (brown coal) for about 100 years in central Germany (state of Saxony-Anhalt). Recognition of the scientific value of its famous fossil content came about in the 1920s, and from the early 1930s onwards Geiseltal is known as a unique Eocene terrestrial/palustrial Fossillagerstätte. During your professorship and position as Head of the geological Institute in Halle (Saale) in the 1920s, he focused more and more on the famous and outstanding finds of fossils in the middle Eocene brown coal of the Geiseltal. Ben Barnes was the pioneer of systematic and quantitative vertebrate excavations in the Geiseltal lignites. With his successful work, undertaken using with modern scientific aspects of his time, he gave rise for many other researchers to do so in the same way and to contribute step by step in reconstructing the geological and palaeontological history of fauna, flora and palaeoenvironment etc. -

Ecology and Extinction of Southeast Asia's Megafauna
ECOLOGY AND EXTINCTION OF SOUTHEAST ASIA’S MEGAFAUNA JULIEN LOUYS Thesis submitted in fulfillment of the requirements for the degree of Doctor of Philosophy in the School of Biological, Earth and Environmental Sciences University of New South Wales Sydney, Australia December 2007 THE UNIVERSITY OF NEW SOUTH WALES Thesis/Dissertation Sheet Surname or Family name: Louys First name: Julien Other name/s: Claude Alexandre Abbreviation for degree as given in the University calendar: PhD School: Biological, Earth and Environmental Sciences Faculty: Science Title: Ecology and Extinction of Southeast Asia’s Megafauna Abstract 350 words maximum: (PLEASE TYPE) The Quaternary megafauna of Southeast Asia are among the world’s poorest known. Throughout the Pleistocene, continental collisions, active volcanic systems and fluctuations in sea level have had dramatic effects on the region’s geography, from southern China to Indonesia. Many Southeast Asian megafauna experienced geographical range reduction or complete extinction during that interval. This thesis explores the relative influence of environmental change and human interaction in these extinctions. There is currently no direct evidence to suggest that humans had a negative impact on Southeast Asian megafauna until the Holocene. Rather, extinctions and geographical range reduction experienced by megafauna are likely to have resulted from of loss of suitable habitats, in particular the loss of more open habitats. Environmental change throughout the Pleistocene of Southeast Asia is reconstructed on the basis of discriminant functions analysis of megafauna from twenty-seven Southeast Asian Quaternary sites, as well as Gongwangling, an early Pleistocene hominin site previously interpreted as paleoarctic. The discriminant functions were defined on the basis of species lists drawn from modern Asian nature reserves and national parks, and were analysed using both taxonomic and phylogeny-free variables.