Banner – University Health Plans AHCCCS Prior Authorization Guidelines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table 2. 2012 AGS Beers Criteria for Potentially
Table 2. 2012 AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults Strength of Organ System/ Recommendat Quality of Recomm Therapeutic Category/Drug(s) Rationale ion Evidence endation References Anticholinergics (excludes TCAs) First-generation antihistamines Highly anticholinergic; Avoid Hydroxyzin Strong Agostini 2001 (as single agent or as part of clearance reduced with e and Boustani 2007 combination products) advanced age, and promethazi Guaiana 2010 Brompheniramine tolerance develops ne: high; Han 2001 Carbinoxamine when used as hypnotic; All others: Rudolph 2008 Chlorpheniramine increased risk of moderate Clemastine confusion, dry mouth, Cyproheptadine constipation, and other Dexbrompheniramine anticholinergic Dexchlorpheniramine effects/toxicity. Diphenhydramine (oral) Doxylamine Use of diphenhydramine in Hydroxyzine special situations such Promethazine as acute treatment of Triprolidine severe allergic reaction may be appropriate. Antiparkinson agents Not recommended for Avoid Moderate Strong Rudolph 2008 Benztropine (oral) prevention of Trihexyphenidyl extrapyramidal symptoms with antipsychotics; more effective agents available for treatment of Parkinson disease. Antispasmodics Highly anticholinergic, Avoid Moderate Strong Lechevallier- Belladonna alkaloids uncertain except in Michel 2005 Clidinium-chlordiazepoxide effectiveness. short-term Rudolph 2008 Dicyclomine palliative Hyoscyamine care to Propantheline decrease Scopolamine oral secretions. Antithrombotics Dipyridamole, oral short-acting* May -

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

Preventive Report Appendix
Title Authors Published Journal Volume Issue Pages DOI Final Status Exclusion Reason Nasal sumatriptan is effective in treatment of migraine attacks in children: A Ahonen K.; Hamalainen ML.; Rantala H.; 2004 Neurology 62 6 883-7 10.1212/01.wnl.0000115105.05966.a7 Deemed irrelevant in initial screening Seasonal variation in migraine. Alstadhaug KB.; Salvesen R.; Bekkelund SI. Cephalalgia : an 2005 international journal 25 10 811-6 10.1111/j.1468-2982.2005.01018.x Deemed irrelevant in initial screening Flunarizine, a calcium channel blocker: a new prophylactic drug in migraine. Amery WK. 1983 Headache 23 2 70-4 10.1111/j.1526-4610.1983.hed2302070 Deemed irrelevant in initial screening Monoamine oxidase inhibitors in the control of migraine. Anthony M.; Lance JW. Proceedings of the 1970 Australian 7 45-7 Deemed irrelevant in initial screening Prostaglandins and prostaglandin receptor antagonism in migraine. Antonova M. 2013 Danish medical 60 5 B4635 Deemed irrelevant in initial screening Divalproex extended-release in adolescent migraine prophylaxis: results of a Apostol G.; Cady RK.; Laforet GA.; Robieson randomized, double-blind, placebo-controlled study. WZ.; Olson E.; Abi-Saab WM.; Saltarelli M. 2008 Headache 48 7 1012-25 10.1111/j.1526-4610.2008.01081.x Deemed irrelevant in initial screening Divalproex sodium extended-release for the prophylaxis of migraine headache in Apostol G.; Lewis DW.; Laforet GA.; adolescents: results of a stand-alone, long-term open-label safety study. Robieson WZ.; Fugate JM.; Abi-Saab WM.; 2009 Headache 49 1 45-53 10.1111/j.1526-4610.2008.01279.x Deemed irrelevant in initial screening Safety and tolerability of divalproex sodium extended-release in the prophylaxis of Apostol G.; Pakalnis A.; Laforet GA.; migraine headaches: results of an open-label extension trial in adolescents. -

Conjugation of Tryptamine to Biologically Active Carboxylic Acids in Order to Create Prodrugs
ISSN 2380-5064 | The Arsenal is published by the Augusta University Libraries | http://guides.augusta.edu/arsenal Volume 4, Issue 1 (2021) Special Edition Issue CONJUGATION OF TRYPTAMINE TO BIOLOGICALLY ACTIVE CARBOXYLIC ACIDS IN ORDER TO CREATE PRODRUGS Colin Miller, Jr. and Iryna O. Lebedyeva Citation Miller, C., Jr., & Lebedyeva, I. O. (2021). Conjugation of tryptamine to biologically active carboxylic acids in order to create prodrugs. The Arsenal: The Undergraduate Research Journal of Augusta University, 4(1), 26. http://doi.org/10.21633/issn.2380.5064/s.2021.04.01.26 © Miller, Jr. and Lebedyeva 2021. This open access article is distributed under a Creative Commons Attribution NonCommercial-NoDerivs 2.0 Generic License (https://creativecommons.org/licenses/by-nc-nd/2.0/). Conjugation of Tryptamine to Biologically Active Carboxylic Acids in Order to Create Prodrugs Presenter(s): Colin Miller, Jr. Author(s): Colin Miller Jr. and Iryna O. Lebedyeva Faculty Sponsor(s): Iryna O. Lebedyeva, PhD Affiliation(s): Department of Chemistry and Physics (Augusta Univ.) ABSTRACT A number of blood and brain barrier penetrating neurotransmitters contain polar functional groups. Gamma-aminobutyric acid (GABA) is an amino acid, which is one of the primary inhibitory neurotransmitter in the brain and a major inhibitory neurotransmitter in the spinal cord. Antiepileptic medications such as Gabapentin, Phenibut, and Pregabalin have been developed to structurally represent GABA. These drugs are usually prescribed for the treatment of neuropathic pain. Since these drugs contain a polar carboxylic acid group, it affects their ability to penetrate the blood and brain barrier. To address the low bioavailability and tendency for intramolecular cyclization of Gabapentin, its less polar prodrug Gabapentin Enacarbil has been approved in 2011. -

(12) United States Patent (10) Patent No.: US 8,283,487 B2 Ben Moha-Lerman Et Al
USOO8283487B2 (12) United States Patent (10) Patent No.: US 8,283,487 B2 Ben Moha-Lerman et al. (45) Date of Patent: Oct. 9, 2012 (54) PROCESSES FOR THE PREPARATION AND 2003/0176398 A1 9/2003 Gallop et al. PURIFICATION OF GABAPENTIN 2004/0077553 A1* 4/2004 Gallop et al. ................... 514, 19 2005/O154057 A1 7/2005 Estrada et al. ENACARBL 2007/0049627 A1 3, 2007 Tran (75) Inventors: Elena Ben Moha-Lerman, Kiryat Ono FOREIGN PATENT DOCUMENTS (IL); Tamar Nidam, Yehud (IL); Meital WO WO O2/10O347 12/2002 Cohen, Petach-Tikva (IL); Sharon WO WO 2005/0377.84 4/2005 Avhar-Maydan, Givataym (IL); Anna Balanov, Rehovot (IL) OTHER PUBLICATIONS X. Yuan et al. “In Situ Preparation of Zinc Salts of Unsaturated (73) Assignee: Teva Pharmaceutical Industries Ltd., Carboxylic Acids to Reinforce NBR' J. Applied Polymer Science, Petach-Tikva (IL) vol. 77, p. 2740-2748 (2000). - r J. Alexander et al. “(Acyloxy)alkyl Carbamates as Novel Biorevers (*) Notice: Subject to any disclaimer, the term of this ible Prodrugs for Amines: Increased Permeation through Biological patent is extended or adjusted under 35 Membranes”.J. Med. Chem. 1988, 31, pp. 318-322. U.S.C. 154(b) by 249 days. S.M. Rahmathullah et al. “Prodrugs for Amidines: Synthesis and Anti-Pneumocystis carinii Activity of Carbamates of 2.5-Bis(4- (21) Appl. No.: 12/626,682 amidinophenyl)furan” J. Med. Chem. 1999, 42, pp. 3994-4000. (22) Filed: Nov. 26, 2009 * cited by examiner (65) Prior Publication Data Primary Examiner — Joseph K. McKane US 2010/O160665 A1 Jun. 24, 2010 Assistant Examiner — Alicia L Otton (74) Attorney, Agent, or Firm — Arent Fox LLP Related U.S. -

More Treatments on Deck for Alcohol Use Disorder
News & Analysis Medical News & Perspectives More Treatments on Deck for Alcohol Use Disorder Jeff Lyon hirteen years have passed since the Raye Z. Litten, PhD, acting director of the ment for AUD were actually prescribed an US Food and Drug Administration NIAAA’s Division of Medications Develop- AUD medication. T (FDA) last approved a new medica- ment and principal investigator of the trial, It is a complicated problem, says John tion to help the nation’s millions of people says the agency is “excited.” Rotrosen, MD, a psychiatrist and addiction withalcoholusedisorder(AUD)stopormod- Nevertheless, the science of alcohol ad- specialist at New York University School of erate their drinking. diction has seen its share of medicines that Medicine. “To a large extent primary care Only 3 such formulations exist, and 1, failed to live up to their early promise dur- physicians don’t screen for alcoholism,” he disulfiram, dates to the Prohibition era. ing full-scale testing. So Litten said the said. “When they do, they may note it, but Known commercially as Antabuse and agency isn’t putting all its eggs in one bas- don’t really do anything about it.” introduced in 1923, it makes people memo- ket. “You need as many weapons as pos- As far as the failure to prescribe exist- rably ill if they ingest alcohol, but it doesn’t sible to treat a complex disease like alcohol ing medications for AUD, Rotrosen blames stop the cravings. The other 2, naltrexone use disorder,” he said. a lack of knowledge. “A lot of doctors in and acamprosate, approved in 1994 and But therein lies a second difficulty. -

A Comparative Effectiveness Meta-Analysis of Drugs for the Prophylaxis of Migraine Headache
University of South Florida Masthead Logo Scholar Commons School of Information Faculty Publications School of Information 7-2015 A Comparative Effectiveness Meta-Analysis of Drugs for the Prophylaxis of Migraine Headache Authors: Jeffrey L. Jackson, Elizabeth Cogbill, Rafael Santana-Davila, Christina Eldredge, William Collier, Andrew Gradall, Neha Sehgal, and Jessica Kuester OBJECTIVE: To compare the effectiveness and side effects of migraine prophylactic medications. DESIGN: We performed a network meta-analysis. Data were extracted independently in duplicate and quality was assessed using both the JADAD and Cochrane Risk of Bias instruments. Data were pooled and network meta-analysis performed using random effects models. DATA SOURCES: PUBMED, EMBASE, Cochrane Trial Registry, bibliography of retrieved articles through 18 May 2014. ELIGIBILITY CRITERIA FOR SELECTING STUDIES: We included randomized controlled trials of adults with migraine headaches of at least 4 weeks in duration. RESULTS: Placebo controlled trials included alpha blockers (n = 9), angiotensin converting enzyme inhibitors (n = 3), angiotensin receptor blockers (n = 3), anticonvulsants (n = 32), beta-blockers (n = 39), calcium channel blockers (n = 12), flunarizine (n = 7), serotonin reuptake inhibitors (n = 6), serotonin norepinephrine reuptake inhibitors (n = 1) serotonin agonists (n = 9) and tricyclic antidepressants (n = 11). In addition there were 53 trials comparing different drugs. Drugs with at least 3 trials that were more effective than placebo for episodic migraines -
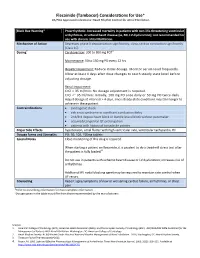
Flecainide Considerations For
Flecainide (Tambocor) Considerations for Use* US/FDA Approved Indications: Heart Rhythm Control for Atrial Fibrillation Black Box Warning* Proarrhythmic. Increased mortality in patients with non-life-threatening ventricular arrhythmias, structural heart disease (ie, MI, LV dysfunction); not recommended for use with chronic atrial fibrillation. Mechanism of Action Depresses phase 0 depolarization significantly, slows cardiac conduction significantly (Class 1C). Dosing† Cardioversion: 200 to 300 mg PO‡1 Maintenance: 50 to 150 mg PO every 12 hrs Hepatic Impairment: Reduce initial dosage. Monitor serum level frequently. Allow at least 4 days after dose changes to reach steady state level before adjusting dosage. Renal Impairment: CrCl > 35 ml/min: No dosage adjustment is required. CrCl <= 35 ml/min: Initially, 100 mg PO once daily or 50 mg PO twice daily. Adjust dosage at intervals > 4 days, since steady-state conditions may take longer to achieve in these patient Contraindications cardiogenic shock sick sinus syndrome or significant conduction delay 2nd/3rd degree heart block or bundle brand block without pacemaker acquired/congenital QT prolongation patients with history of torsade de pointes Major Side Effects hypotension, atrial flutter with high ventricular rate, ventricular tachycardia, HF Dosage forms and Strengths PO: 50, 100, 150mg tablets Special Notes Close monitoring of this drug is required. When starting a patient on flecainide, it is prudent to do a treadmill stress test after the patient is fully loaded.4 Do not use in patients with ischemic heart disease or LV dysfunction; increases risk of arrhythmias. Additional AV nodal blocking agent may be required to maintain rate control when AF recurs. -

A Proposal for the Clinical Use of Flecainide
A Proposal for the Clinical Use of Flecainide JEFFREY L. ANDERSON, MD, JAMES R. STEWART, MD, and BARRY J. CREVEY, MD Effective antiarrhythmic therapy requires a carefully sponse rate is observed in preventing induction of considered approach, including an understanding sustained ventricular tachycardia, and these pa- of the arrhythmia, the underlying cardiac disease tients should be carefully selected. Flecainide is and the drug’s pharmacokinetics. Flecainide is a promising in the treatment of supraventricular new antiarrhythmic drug that may soon be released tachycardias using atrioventricular nodal or extra- for general use. Flecainide demonstrates unsur- nodal reentrant pathways, although this use is still passed efficacy in chronic ventricular arrhythmias investigational in the United States. The drug’s use in stable patients and may become a first-choice for arrhythmias during acute myocardial infarction drug because of its ease of administration, efficacy requires further study. Flecainide possesses modest and favorable tolerance. Twice-daily dosing with negative inotropic potential. Proarrhythmic or other 100 to 200 mg usually provides effective therapy. adverse reactions have occurred primarily in set- Clinical experience suggests flecainide to be indi- tings of high drug level, poor ventricular function cated in the treatment of uniform and multiform or refractory, malignant arrhythmias, suggesting ventricular premature complexes, coupled ven- caution in these groups. tricular premature complexes, and episodes of nonsustained -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -

Gabapentinoids: Pharmacokinetics, Pharmacodynamics and Considerations for Clinical Practice
912496BJP British Journal of PainChincholkar Special Issue Article British Journal of Pain 1 –11 Gabapentinoids: pharmacokinetics, © The British Pain Society 2020 Article reuse guidelines: sagepub.com/journals-permissions pharmacodynamics and considerations DOI:https://doi.org/10.1177/2049463720912496 10.1177/2049463720912496 for clinical practice journals.sagepub.com/home/bjp Mahindra Chincholkar Abstract The gabapentinoids are often recommended as first-line treatments for the management of neuropathic pain. The differing pharmacodynamic and pharmacokinetic profiles can have implications for clinical practice. This article has summarised these key differences. In addition to their use in managing neuropathic pain, gabapentinoids are increasingly being used for off-label conditions despite the lack of evidence. Prescription rates for off-label conditions have overtaken that for on-label use. Similarly, the use of gabapentinoids in the perioperative period is now embedded in clinical practice despite conflicting evidence. This article summarises the risks associated with this increasing use. There is increasing evidence of the potential to cause harm in vulnerable populations such as the elderly and increasing prevalence of abuse. The risk of respiratory depression in combination with opioids is of particular concern in the context of the current opioid crisis. This article describes the practical considerations involved that might help guide appropriate prescribing practices. Keywords Gabapentin, pregabalin, pain management, adverse effects, pharmacology Introduction movement, sleep disorders, headaches, alcohol with- drawal syndrome, chronic back pain, fibromyalgia, vis- The gabapentinoid drugs gabapentin and pregabalin ceral pain and acute postoperative pain.4,5 The rate of are antiepileptic drugs that are considered as first-line new prescriptions is increasing and tripled in England 1 treatments for the management of neuropathic pain. -

Gabapentin Enacarbil (Horizant) Monograph
Gabapentin enacarbil (Horizant) Monograph ® Gabapentin Enacarbil (Horizant ) National Drug Monograph March 2012 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives The purpose of VA PBM Services drug monographs is to provide a comprehensive drug review for making formulary decisions. These documents will be updated when new clinical data warrant additional formulary discussion. Documents will be placed in the Archive section when the information is deemed to be no longer current. Executive Summary: Gabapentin enacarbil (Horizant®) is a prodrug of gabapentin which binds with high affinity to the α2δ subunit of voltage-activated calcium channels in vitro studies. It is unknown how the binding of gabapentin enacarbil (GEn) to the α2δ subunit corresponds to the treatment of restless leg syndrome (RLS) symptoms. Indication: GEn is FDA approved for treatment of RLS. Pharmacokinetics: GEn is primarily excreted by the kidneys and neither gabapentin nor GEn is substrate, inhibitor, or inducer of the major CYP450 system. In addition, GEn is not an inhibitor or substrate of p-glycoprotein in vitro. Dose: The recommended dosage is 600 mg once daily taken with food at about 5 PM. Efficacy: Several studies examining GEn have shown efficacy over placebo in patients with RLS; improvement in RLS symptoms was observed within 7 days of starting treatment.6-8, 12-14 Three studies demonstrated 1200 mg/day GEn significantly reduced International Restless Legs Syndrome (IRLS) scale total score and improved Clinical Global Impression– Improvement (CGI-I) scale compared with placebo.6-8 Two studies demonstrated efficacy at lower dose of 600 mg/day GEn; however, another study did not.7,12,13 These results are comparable to those seen with dopamine agonists.8,12 Adverse Drug Events: Commonly reported adverse effects for the approved dose of 600 mg/day GEn are somnolence, sedation and dizziness.