Forest & Bird, BFG Methodology Wild Fisheries & Freshwater
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
World Bank Document
GLOBAL ENVIRONMENT 33977 FACILITY Public Disclosure Authorized Public Disclosure Authorized Quarterly Operational Report April 1995 Public Disclosure Authorized GEF Public Disclosure Authorized development,agencies, national institutions, (GEF) is a financial tions, bilateral T mechanismhe Global Environment that provides Facility grant and concessional funds non-governmental organizations (NGOs), private sector to developing countries for projects and activities that aim entities, and academic institutions. The GEF also comprises to protect the global environment. GEF resources are avail- a Small Grants Programme available for projects in the able for projects and other activities that address climate four focal areas that are put forward by grassroots groups change, loss of biological diversity, pollution of international and NGOs in developing countries. waters, and depletion of the ozone layer. Countries can The Quarterly Operational Report is designed to pro- obtain GEF funds if they are eligible to borrow from the vide a comprehensive review of, and a status report on, the World Bank (IBRD and/or IDA) or receive technical assis- GEE work program. A brief description of each of the GEE's tance grants from UNDP through a country program. projects organized alphabetically by region can be Responsibility for implementing GEF activities is found on pages 8-J8. Each description lists the name of the shared by the United Nations Development Programme UNDP, UNEP or World Bank Task Manager responsible for (UNDP), the United Nations Environment Programme the project. Inquiries about specific projects should be (UNEP) and the World Bank. UNDP is responsible for referred to the responsible Task Manager. Their telephone technical assistance activities, capacity building, and the and fax numbers can be found on pages 63 and 64. -

A Global Assessment of Parasite Diversity in Galaxiid Fishes
diversity Article A Global Assessment of Parasite Diversity in Galaxiid Fishes Rachel A. Paterson 1,*, Gustavo P. Viozzi 2, Carlos A. Rauque 2, Verónica R. Flores 2 and Robert Poulin 3 1 The Norwegian Institute for Nature Research, P.O. Box 5685, Torgarden, 7485 Trondheim, Norway 2 Laboratorio de Parasitología, INIBIOMA, CONICET—Universidad Nacional del Comahue, Quintral 1250, San Carlos de Bariloche 8400, Argentina; [email protected] (G.P.V.); [email protected] (C.A.R.); veronicaroxanafl[email protected] (V.R.F.) 3 Department of Zoology, University of Otago, P.O. Box 56, Dunedin 9054, New Zealand; [email protected] * Correspondence: [email protected]; Tel.: +47-481-37-867 Abstract: Free-living species often receive greater conservation attention than the parasites they support, with parasite conservation often being hindered by a lack of parasite biodiversity knowl- edge. This study aimed to determine the current state of knowledge regarding parasites of the Southern Hemisphere freshwater fish family Galaxiidae, in order to identify knowledge gaps to focus future research attention. Specifically, we assessed how galaxiid–parasite knowledge differs among geographic regions in relation to research effort (i.e., number of studies or fish individuals examined, extent of tissue examination, taxonomic resolution), in addition to ecological traits known to influ- ence parasite richness. To date, ~50% of galaxiid species have been examined for parasites, though the majority of studies have focused on single parasite taxa rather than assessing the full diversity of macro- and microparasites. The highest number of parasites were observed from Argentinean galaxiids, and studies in all geographic regions were biased towards the highly abundant and most widely distributed galaxiid species, Galaxias maculatus. -

The Osmoregulatory Metabolism Op the Starry Flounder, Platichthys Stellatus
THE OSMOREGULATORY METABOLISM OP THE STARRY FLOUNDER, PLATICHTHYS STELLATUS by CLEVELAND PENDLETON HICKMAN, JR. B.A., DePauw University, 1950 M.S., University of New Hampshire, 1953 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in the Department of Zoology We accept this thesis as conforming to the required standard. THE UNIVERSITY OF BRITISH COLUMBIA June, 1958 Faculty of Graduate Studies PROGRAMME OF THE FINAL ORAL EXAMINATION FOR THE DEGREE OF DOCTOR OF PHILOSOPHY of CLEVELAND PENDLETON HICKMAN JR. B.A. DePauw University, 1950 M.S. University of New Hampshire, 1953 IN ROOM 187A, BIOLOGICAL SCIENCES BUILDING MONDAY, JUNE 30, 1958 at 10:30 a.m. COMMITTEE IN CHARGE DEAN F. H. SOWARD, Chairman H. ADASKIN W. S. HOAR W. A. CLEMENS W. N. HOLMES I. McT. COWAN C. C. LINDSEY P. A. DEHNEL H. McLENNAN R. F. SCAGEL External Examiner: F. E. J. FRY University of Toronto THE OSMOREGULATORY METABOLISM OF THE STARRY FLOUNDER, PLATICHTYS STELLATUS ABSTRACT Energy demands for osmotic regulation and the possible osmoregulatory role of the thyroid gland were investigated in the euryhaline starry flounder, Platichthys stellatus. Using a melt• ing-point technique, it was established that flounder could regulate body fluid concentration independent of widely divergent environ• mental salinities. Small flounder experienced more rapid disturb• ances of body fluid concentration than large flounder after abrupt salinity alterations. The standard metabolic rate of flounder adapted to fresh water was consistently and significantly less than that of marine flounder. In supernormal salinities standard metabolic rate was significantly greater than in normal sea water. -

Marine Fish Conservation Global Evidence for the Effects of Selected Interventions
Marine Fish Conservation Global evidence for the effects of selected interventions Natasha Taylor, Leo J. Clarke, Khatija Alliji, Chris Barrett, Rosslyn McIntyre, Rebecca0 K. Smith & William J. Sutherland CONSERVATION EVIDENCE SERIES SYNOPSES Marine Fish Conservation Global evidence for the effects of selected interventions Natasha Taylor, Leo J. Clarke, Khatija Alliji, Chris Barrett, Rosslyn McIntyre, Rebecca K. Smith and William J. Sutherland Conservation Evidence Series Synopses 1 Copyright © 2021 William J. Sutherland This work is licensed under a Creative Commons Attribution 4.0 International license (CC BY 4.0). This license allows you to share, copy, distribute and transmit the work; to adapt the work and to make commercial use of the work providing attribution is made to the authors (but not in any way that suggests that they endorse you or your use of the work). Attribution should include the following information: Taylor, N., Clarke, L.J., Alliji, K., Barrett, C., McIntyre, R., Smith, R.K., and Sutherland, W.J. (2021) Marine Fish Conservation: Global Evidence for the Effects of Selected Interventions. Synopses of Conservation Evidence Series. University of Cambridge, Cambridge, UK. Further details about CC BY licenses are available at https://creativecommons.org/licenses/by/4.0/ Cover image: Circling fish in the waters of the Halmahera Sea (Pacific Ocean) off the Raja Ampat Islands, Indonesia, by Leslie Burkhalter. Digital material and resources associated with this synopsis are available at https://www.conservationevidence.com/ -

A Freshwater Fish Database: a User Guide for DOC Users
SCIENCE & RESEARCH INTERNAL REPORT NO.127 THE FRESHWATER FISH DATABASE: A USER GUIDE FOR DOC USERS by Jody Richardson and Helen Adcock This is an internal Department of Conservation report and must be cited as Science and Research Internal Report No.127. Permission to use any of its contents must be obtained from the Director (Science & Research), Head Office, Department of Conservation. Published by Head Office, Department of Conservation, P O Box 10-420, Wellington New Zealand ISSN 0114-2798 ISBN 0-478-01407-4 CONTENTS ABSTRACT 1 1. INTRODUCTION 1 2. BACKGROUND 1 3. HOW TO EXTRACT DATA 2 3.1 How to use the menus 3 3.2 Menu options 3 3.2.1 List species info 3 3.2.2 Next record 3 3.2.3 Previous record 3 3.2.4 Go to record no 4 3.2.5 Search records 4 3.2.6 List site records 4 3.2.7 Print record 5 3.2.8 Sort records 5 3.2.9 Clear 5 3.2.10 Quit 5 4. DATA INTERPRETATION 6 5. A FEW RULES ABOUT DATA USE 7 APPENDIX I. - ORIGINAL DATABASE CARD (1978) 9 APPENDIX II - REVISED DATABASE CARD (1986) 10 APPENDIX III - DATABASE COVERAGE FOR THE NORTH AND SOUTH ISLANDS, JANUARY 1992 11 APPENDIX IV - ECOLOGICAL REGIONS AND DISTRICTS OF NEW ZEALAND, 3rd Edition 12 APPENDIX V - LOCAL AUTHORITIES OF NEW ZEALAND, 1992 14 APPENDIX VI - DEPARTMENT OF CONSERVATION BOUNDARIES, 1992 16 APPENDIX VII - FISH SPECIES LIST (sorted on scientific name) 18 APPENDIX VIII - FISH SPECIES LIST (sorted on common name) 20 APPENDIX IX - FISHING METHODS 22 APPENDIX X - MEMORANDUM OF AGREEMENT 23 THE FRESHWATER FISH DATABASE A User Guide for DOC Users by Jody Richardson1 and Helen Adcock2 1National Institute of Water & Atmospheric Research Ltd. -

Wetlands, Biodiversity and the Ramsar Convention
Wetlands, Biodiversity and the Ramsar Convention Wetlands, Biodiversity and the Ramsar Convention: the role of the Convention on Wetlands in the Conservation and Wise Use of Biodiversity edited by A. J. Hails Ramsar Convention Bureau Ministry of Environment and Forest, India 1996 [1997] Published by the Ramsar Convention Bureau, Gland, Switzerland, with the support of: • the General Directorate of Natural Resources and Environment, Ministry of the Walloon Region, Belgium • the Royal Danish Ministry of Foreign Affairs, Denmark • the National Forest and Nature Agency, Ministry of the Environment and Energy, Denmark • the Ministry of Environment and Forests, India • the Swedish Environmental Protection Agency, Sweden Copyright © Ramsar Convention Bureau, 1997. Reproduction of this publication for educational and other non-commercial purposes is authorised without prior perinission from the copyright holder, providing that full acknowledgement is given. Reproduction for resale or other commercial purposes is prohibited without the prior written permission of the copyright holder. The views of the authors expressed in this work do not necessarily reflect those of the Ramsar Convention Bureau or of the Ministry of the Environment of India. Note: the designation of geographical entities in this book, and the presentation of material, do not imply the expression of any opinion whatsoever on the part of the Ranasar Convention Bureau concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Citation: Halls, A.J. (ed.), 1997. Wetlands, Biodiversity and the Ramsar Convention: The Role of the Convention on Wetlands in the Conservation and Wise Use of Biodiversity. -

Freshwater Fish Spawning and Migration Periods
Freshwater Fish Spawning and Migration Periods Prepared for Ministry for Primary Industries November 2014 Prepared by: Josh Smith For any information regarding this report please contact: Josh Smith Freshwater Fish Technician Freshwater & Estuaries Phone +64 07 8567026 [email protected] National Institute of Water & Atmospheric Research Ltd Gate 10 Silverdale Road Hillcrest, Hamilton 3216 PO Box 11115, Hillcrest Hamilton 3251 New Zealand Phone +64 07 8567026 NIWA CLIENT REPORT No: HAM2014-101 Report date: November 2014 NIWA Project: MPI15202 ISBN 978-0-473-32827-6 © All rights reserved. This publication may not be reproduced or copied in any form without the permission of the copyright owner(s). Such permission is only to be given in accordance with the terms of the client’s contract with NIWA. This copyright extends to all forms of copying and any storage of material in any kind of information retrieval system. Whilst NIWA has used all reasonable endeavours to ensure that the information contained in this document is accurate, NIWA does not give any express or implied warranty as to the completeness of the information contained herein, or that it will be suitable for any purpose(s) other than those specifically contemplated during the Project or agreed by NIWA and the Client. Contents Executive summary ............................................................................................................... 6 1 Introduction ............................................................................................................... -

Akbaripasandabbas2012phd.Pdf (1.900Mb)
Habitat use, growth and movement in relation to bioenergetics of drift-feeding stream fish Abbas Akbaripasand A thesis submitted for the degree of Doctor of Philosophy at the University of Otago, Dunedin, New Zealand September 2012 ABSTRACT The distribution and abundance of fish species is influenced by a range of environmental variables. Information on driving factors in that determines fish habitat use, abundance and distribution is crucial for fisheries and restoration management, especially for species in gradual decline. Habitat use may be structured by interactions between multiple factors including food density, predation risk, competitors, energetic requirements and habitat structure. Habitat selection varies with respect to species, stage of life history and time of day, agonistic interactions between conspecifics may also influence habitat use. In this study, I selected three streams draining to Otago Harbour in the South Island, New Zealand; Craigs Creek, Deborah Bay Stream and Sawyers Bay Stream. A gradient in riparian vegetation from sparse (Craigs) to dense (Deborah) was the basis of stream selection and they likely differ in food quantity and quality which may affect fish assemblages. I used banded kokopu (Galaxias fasciatus) as an animal model. The fish represent ideal species to study feeding strategies, growth and movement as they appear to form dominance hierarchies that are stable over long periods of time within stream pools. They are also easy to catch, observe and individually tag. High fish density in Craigs Creek and low food density in Deborah Bay Stream and absence of trout in all streams provides an ideal situation to study the role of social hierarchies and intraspecific competition for food and space on patterns of individual growth, residency and movement. -

Yellow-Eyed Penguin Diet and Indirect Effects
CSP16205-1 POP2016-05 Mattern & Ellenberg - Yellow-eyed penguin diet and indirect effects 1 CSP16205-1 POP2016-05 Mattern & Ellenberg - Yellow-eyed penguin diet and indirect effects Table of Contents Abstract ....................................................................................................3 Introduction .............................................................................................4 Background/context ............................................................................................ 4 Objectives ........................................................................................................... 5 Methods ....................................................................................................5 Results ......................................................................................................6 Main prey species, inter-decadal & regional variations ...................................... 6 Prey sizes, age classes & temporal occurrence .................................................. 11 Differences between adult & juvenile penguins ............................................... 13 Prey behaviour & capture strategies ................................................................. 13 Prey association with benthic sediments .......................................................... 14 Indirect fisheries effects on prey composition and behaviour ........................... 17 Linear foraging along bottom trawl furrows .................................................... -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

Effect of Diet and Sex-Sorting on Growth and Gonad Development in Farmed South African Abalone, Haliotis Midae
Effect of diet and sex-sorting on growth and gonad development in farmed South African abalone, Haliotis midae A thesis submitted in fulfilment of the requirements for the degree of MASTER OF SCIENCE of RHODES UNIVERSITY By DEVIN WILLIAM PHILIP AYRES December 2013 ABSTRACT Abalone, Haliotis midae, farmers in South Africa that feed formulated diets reported a periodic drop in abalone growth during periods of increased gonad development. A large drop in abalone biomass was noticed after presumed spawning events. This study was aimed to determine the effect of diet and sex-sorting on gonad development in abalone. Experiments were conducted on a commercial abalone farm from July 2012 to the end of June 2013. Isonitrogenous and isoenergetic diets were formulated with two protein sources. A fishmeal and soybean meal (S-diet) diet and a fishmeal only (F-diet) diet were fed to abalone (50 - 70 g abalone-1) over 12 months. Weight and length gain, gonad bulk index (GBI), visceral index (%) and meat mass index (%) were determined monthly and seasonally. A histological study on the female gonads was conducted. This study also included an experiment to test the effect of sex-sorting (70 - 80 g abalone-1) on growth and body composition with treatments including males (M), females (F) and equal numbers of males and females (MF). Weight gain and length gain were faster in S-diet-fed abalone (RM-ANOVA, F (1, 16) = 7.77, p = 0.01; F (1, 69) = 49.9, p < 0.001, respectively). Gonad development was significantly affected by the inclusion of soybean meal with S-diet-fed abalone showing higher GBI-values than F- diet-fed abalone (RM-ANOVA, F (1, 33) = 16.22, p = 0.0003). -
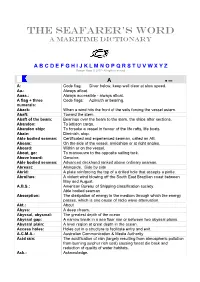
Dictionary.Pdf
THE SEAFARER’S WORD A Maritime Dictionary A B C D E F G H I J K L M N O P Q R S T U V W X Y Z Ranger Hope © 2007- All rights reserved A ● ▬ A: Code flag; Diver below, keep well clear at slow speed. Aa.: Always afloat. Aaaa.: Always accessible - always afloat. A flag + three Code flags; Azimuth or bearing. numerals: Aback: When a wind hits the front of the sails forcing the vessel astern. Abaft: Toward the stern. Abaft of the beam: Bearings over the beam to the stern, the ships after sections. Abandon: To jettison cargo. Abandon ship: To forsake a vessel in favour of the life rafts, life boats. Abate: Diminish, stop. Able bodied seaman: Certificated and experienced seaman, called an AB. Abeam: On the side of the vessel, amidships or at right angles. Aboard: Within or on the vessel. About, go: To manoeuvre to the opposite sailing tack. Above board: Genuine. Able bodied seaman: Advanced deckhand ranked above ordinary seaman. Abreast: Alongside. Side by side Abrid: A plate reinforcing the top of a drilled hole that accepts a pintle. Abrolhos: A violent wind blowing off the South East Brazilian coast between May and August. A.B.S.: American Bureau of Shipping classification society. Able bodied seaman Absorption: The dissipation of energy in the medium through which the energy passes, which is one cause of radio wave attenuation. Abt.: About Abyss: A deep chasm. Abyssal, abysmal: The greatest depth of the ocean Abyssal gap: A narrow break in a sea floor rise or between two abyssal plains.