Hemiptera, Reduviidae, Triatominae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Journal of Venomous Animals and Toxins Including Tropical Diseases
Journal of Venomous Animals and Toxins including Tropical Diseases This Provisional PDF corresponds to the article as it appeared upon acceptance. Fully formatted PDF and full text (HTML) versions will be made available soon. Challenges and perspectives of Chagas disease: a review Journal of Venomous Animals and Toxins including Tropical Diseases 2013, 19:34 doi:10.1186/1678-9199-19-34 Paulo Câmara Pereira ([email protected]) Elaine Cristina Navarro ([email protected]) ISSN 1678-9199 Article type Review Submission date 1 November 2013 Acceptance date 5 December 2013 Publication date 19 December 2013 Article URL http://www.jvat.org/content/19/1/34 This peer-reviewed article can be downloaded, printed and distributed freely for any purposes (see copyright notice below). Articles in JVATiTD are listed in PubMed and archived at PubMed Central. For information about publishing your research in JVATiTD or any BioMed Central journal, go to http://www.jvat.org/authors/instructions/ For information about other BioMed Central publications go to http://www.biomedcentral.com/ © 2013 Pereira and Navarro This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. Challenges and perspectives of Chagas disease: a review Paulo Câmara Marques Pereira 1* *Corresponding author Email: [email protected] Elaine Cristina Navarro 1,2 Email: [email protected] 1Department of Tropical Diseases, Botucatu Medical School, São Paulo State University (UNESP – Univ Estadual Paulista), Av. -

When Hiking Through Latin America, Be Alert to Chagas' Disease
When Hiking Through Latin America, Be Alert to Chagas’ Disease Geographical distribution of main vectors, including risk areas in the southern United States of America INTERNATIONAL ASSOCIATION 2012 EDITION FOR MEDICAL ASSISTANCE For updates go to www.iamat.org TO TRAVELLERS IAMAT [email protected] www.iamat.org @IAMAT_Travel IAMATHealth When Hiking Through Latin America, Be Alert To Chagas’ Disease COURTESY ENDS IN DEATH segment upwards, releases a stylet with fine teeth from the proboscis and Valle de los Naranjos, Venezuela. It is late afternoon, the sun is sinking perforates the skin. A second stylet, smooth and hollow, taps a blood behind the mountains, bringing the first shadows of evening. Down in the vessel. This feeding process lasts at least twenty minutes during which the valley a campesino is still tilling the soil, and the stillness of the vinchuca ingests many times its own weight in blood. approaching night is broken only by a light plane, a crop duster, which During the feeding, defecation occurs contaminating the bite wound periodically flies overhead and disappears further down the valley. with feces which contain parasites that the vinchuca ingested during a Bertoldo, the pilot, is on his final dusting run of the day when suddenly previous bite on an infected human or animal. The irritation of the bite the engine dies. The world flashes before his eyes as he fights to clear the causes the sleeping victim to rub the site with his or her fingers, thus last row of palms. The old duster rears up, just clipping the last trees as it facilitating the introduction of the organisms into the bloodstream. -

Major Article Natural Infection by Trypanosoma Cruzi in Triatomines
Rev Soc Bras Med Trop 51(2):190-197, March-April, 2018 doi: 10.1590/0037-8682-0088-2017 Major Article Natural infection by Trypanosoma cruzi in triatomines and seropositivity for Chagas disease of dogs in rural areas of Rio Grande do Norte, Brazil Yannara Barbosa Nogueira Freitas[1], Celeste da Silva Freitas de Souza[2], Jamille Maia e Magalhães[1], Maressa Laíse Reginaldo de Sousa[1], Luiz Ney d’Escoffier[2], Tânia Zaverucha do Valle[2], Teresa Cristina Monte Gonçalves[3], Hélcio Reinaldo Gil-Santana[4], Thais Aaparecida Kazimoto[1] and Sthenia Santos Albano Amora[1] [1]. Centro de Ciências Agrárias, Universidade Federal Rural do Semi-Árido, Mossoró, RN, Brasil. [2]. Laboratório de Imunomodulação e Protozoologia, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz, Rio de Janeiro, RJ, Brasil. [3]. Laboratório Interdisciplinar de Vigilância Entomológica em Diptera e Hemiptera, Fundação Oswaldo Cruz, Rio de Janeiro, RJ, Brasil. [4]. Laboratório de Diptera, Fundação Oswaldo Cruz, Rio de Janeiro, RJ, Brasil. Abstract Introduction: Chagas disease is caused by the protozoa Trypanosoma cruzi. Its main reservoir is the domestic dog, especially in rural areas with favorable characteristics for vector establishment and proliferation. The aims of this study were to collect data, survey and map the fauna, and identify T. cruzi infection in triatomines, as well as to assess the presence of anti-T. cruzi antibodies in dogs in rural areas of the municipality of Mossoró, Brazil. Methods: An active entomologic research was conducted to identify adult specimens through an external morphology dichotomous key. The analysis of natural infection by T. cruzi in the insects was performed by isolation in culture and polymerase chain reaction. -

Brasiliensin: a Novel Intestinal Thrombin Inhibitor from Triatoma Brasiliensis (Hemiptera: Reduviidae) with an Important Role in Blood Intake
International Journal for Parasitology 37 (2007) 1351–1358 www.elsevier.com/locate/ijpara Brasiliensin: A novel intestinal thrombin inhibitor from Triatoma brasiliensis (Hemiptera: Reduviidae) with an important role in blood intake R.N. Araujo a, I.T.N. Campos b, A.S. Tanaka b, A. Santos a, N.F. Gontijo a, M.J. Lehane c, M.H. Pereira a,* a Departamento de Parasitologia, Instituto de Cieˆncias Biolo´gicas, UFMG, Bloco 14, Sala 177, Av. Antoˆnio Carlos 6627, Belo Horizonte, MG, Brazil b Departamento de Bioquı´mica, Escola Paulista de Medicina, UNIFESP-EPM, Sa˜o Paulo, SP, Brazil c Liverpool School of Tropical Medicine, Pembroke Place, Liverpool L3 5QA, UK Received 6 February 2007; received in revised form 16 April 2007; accepted 24 April 2007 Abstract Every hematophagous invertebrate studied to date produces at least one inhibitor of coagulation. Among these, thrombin inhibitors have most frequently been isolated. In order to study the thrombin inhibitor from Triatoma brasiliensis and its biological significance for the bug, we sequenced the corresponding gene and evaluated its biological function. The T. brasiliensis intestinal thrombin inhibitor, termed brasiliensin, was sequenced and primers were designed to synthesize double strand RNA (dsRNA). Gene knockdown (RNAi) was induced by two injections of 15 lg of dsRNA into fourth instar nymphs. Forty-eight hours after the second injection, bugs from each group were allowed to feed on hamsters. PCR results showed that injections of dsRNA reduced brasiliensin expression in the ante- rior midgut by approximately 71% in knockdown nymphs when compared with controls. The reduction in gene expression was con- firmed by the thrombin inhibitory activity assay and the citrated plasma coagulation time assay which showed activity reductions of 18- and 3.5-fold, respectively. -

Vectors of Chagas Disease, and Implications for Human Health1
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Jurberg Jose, Galvao Cleber Artikel/Article: Biology, ecology, and systematics of Triatominae (Heteroptera, Reduviidae), vectors of Chagas disease, and implications for human health 1095-1116 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Biology, ecology, and systematics of Triatominae (Heteroptera, Reduviidae), vectors of Chagas disease, and implications for human health1 J. JURBERG & C. GALVÃO Abstract: The members of the subfamily Triatominae (Heteroptera, Reduviidae) are vectors of Try- panosoma cruzi (CHAGAS 1909), the causative agent of Chagas disease or American trypanosomiasis. As important vectors, triatomine bugs have attracted ongoing attention, and, thus, various aspects of their systematics, biology, ecology, biogeography, and evolution have been studied for decades. In the present paper the authors summarize the current knowledge on the biology, ecology, and systematics of these vectors and discuss the implications for human health. Key words: Chagas disease, Hemiptera, Triatominae, Trypanosoma cruzi, vectors. Historical background (DARWIN 1871; LENT & WYGODZINSKY 1979). The first triatomine bug species was de- scribed scientifically by Carl DE GEER American trypanosomiasis or Chagas (1773), (Fig. 1), but according to LENT & disease was discovered in 1909 under curi- WYGODZINSKY (1979), the first report on as- ous circumstances. In 1907, the Brazilian pects and habits dated back to 1590, by physician Carlos Ribeiro Justiniano das Reginaldo de Lizárraga. While travelling to Chagas (1879-1934) was sent by Oswaldo inspect convents in Peru and Chile, this Cruz to Lassance, a small village in the state priest noticed the presence of large of Minas Gerais, Brazil, to conduct an anti- hematophagous insects that attacked at malaria campaign in the region where a rail- night. -

Chagas Disease in Northeast of Brazil: Findings from a Systematic
ISSN: 2447-2301 REVISÃO / REVIEW / REVISIÓN Chagas disease in Northeast of Brazil: Findings from a systematic review of literature Doença de chagas no Nordeste do Brasil: conclusões de uma revisão sistemática da literatura Enfermedad de chagas en el Noreste de Brasil: hallazgos de una revisión sistemática de la literature Danielle Misael de Sousa ¹ Alice Helena Ricardo-Silva² Simone Patricia Carneiro de Freitas³ Filipe Anibal Carvalho Costa4 Helena Keiko Toma5 Angelita Alves de Carvalho6 Jacenir Reis Dos Santos Mallet7 Descriptors ABSTRACT Among the infectious diseases related to poverty, Chagas' disease is a disease that affects about 6 to 8 million people Intestinal parasites. Neglected in America. Its etiologic agent is the Trypanosoma cruzi protozoan, which is transmitted by hematophagous vectors of the Triatominae (Reduviidae) subfamily. In Brazil, vectorial transmission has declined in recent years. However, it is diseases. Environment. of utmost importance to constantly monitor and control the vectors of this disease, because it is still possible to find Tuberculosis. natural foci of triatomines in all geographic regions of the country. The Northeast of Brazil is the region where Chagas disease occurs endemically and has large outbreaks of transmission and still has the highest number of vectors captured Descritores in Brazil, accounting for more than half of the total attributed to the country. In addition, the Northeast continues to Parasitas intestinais. Doenças be one of the poorest regions of the country, with a large number of houses that proliferatetriatomines. Considering negligenciadas. Ambiente. the importance of the Northeast region for the panorama of Chagas' disease in Brazil, this article makes a systematic Tuberculose. -

Chec List ISSN 1809-127X (Available at Journal of Species Lists and Distribution
Check List 10(4): 944–946, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution N Cavernicola pilosa Barber, 1937 (Hemiptera: Reduviidae: ISTRIBUTIO D Triatominae): 1* First report in the state 3 of Maranhão, Brazil 2 RAPHIC G Hélcio R. Gil-Santana , Cleber Galvão , Carlos G. C. Mielke EO 1 Fundação Oswaldo Cruz, Instituto Oswaldo Cruz, Laboratório de Diptera, and Programa de Pós-Graduação em Biodiversidade e Saúde. Av. Brasil G N 4365. CEP 21040-360. Rio de Janeiro, RJ, Brazil. O 2 Fundação Oswaldo Cruz, Instituto Oswaldo Cruz, Laboratório Nacional e Internacional de Referência em Taxonomia de Triatomíneos. Av. Brasil 4365. CEP 21040-360. Rio de [email protected], RJ, Brazil. ; [email protected] OTES 3 Caixa Postal 1206. CEP 84145-000. Carambeí, PR, Brazil. N * Corresponding Author. E-mail: Abstract: Cavernicola pilosa State, Northeastern Brazil. Barber, 1937 (Hemiptera: Reduviidae: Triatominae) is reported for the first time in Maranhão DOI: 10.15560/10.4.944 et al. Rhodnius domesticus Triatoma sordida Since the discovery of Chagas (1909), blood-sucking (1998), whileet al. Neiva & Pinto, insects of the subfamily TriatominaeTrypanosoma have been cruzi recognized 1923 and (Stål, 1859) were recorded as real or potential vectors of Chagas disease, caused by only by Galvão (2003), thus making a total of 17 infection by the protozoan (Chagas, femalespecies ofalready Cavernicola recorded pilosa in this state (Table 1). 1909) (Lent and Wygodzinsky 1979).Cavernicola Barber, 1937, The material examined consisted of a male and a Among the five tribes included in Triatominae,C. -

Hemiptera: Reduviidae: Triatominae) Jane Costa+, Márcio Felix
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 102(1): 87-90, February 2007 87 Triatoma juazeirensis sp. nov. from the state of Bahia, Northeastern Brazil (Hemiptera: Reduviidae: Triatominae) Jane Costa+, Márcio Felix Laboratório da Coleção Entomológica, Departamento de Entomologia, Instituto Oswaldo Cruz-Fiocruz, Av. Brasil 4365, 21045-900 Rio de Janeiro, RJ, Brasil Triatoma juazeirensis, a new triatomine species from the state of Bahia, Northeastern Brazil, is described. The new species is found among rocks in sylvatic environment and in the peridomicile. Type specimens were deposited in the Entomological Collection of Oswaldo Cruz Institute-Fiocruz, Museum of Zoology of Univer- sity of São Paulo, and Florida Museum of Natural History. T. juazeirensis can be distinguished from the other members of the T. brasiliensis species complex mainly by the overall color of the pronotum, which is dark, and by the entirely dark femora. Key words: Triatoma juazeirensis sp. nov. - Triatoma brasiliensis complex - Chagas disease vector - taxonomy - morphology - Neotropics The genus Triatoma Laporte, 1832 is currently known MATERIAL AND METHODS from 66 species of which 27 have been reported in Bra- The material herein studied is deposited in the zil (Galvão et al. 2003). Triatoma brasiliensis Neiva, Coleção Entomológica, Instituto Oswaldo Cruz-Fiocruz, 1911, the main Chagas disease vector in semiarid areas Rio de Janeiro, Brazil (CEIOC); Museu de Zoologia, of Northeastern Brazil (Silveira & Vinhaes 1999, Costa Universidade de São Paulo, São Paulo, Brazil (MZUSP); et al. 2003a), presents great chromatic variation. This and Florida Museum of Natural History (University of aspect has lead in the past to the description of two mela- Florida), Gainesville, US (FLMNH). -

Characterization of Female External Genitalia and Eggs of Four South American Species of the Triatoma Laporte, 1832 Genus (Hemiptera: Reduviidae: Triatominae)
insects Article Characterization of Female External Genitalia and Eggs of Four South American Species of the Triatoma Laporte, 1832 Genus (Hemiptera: Reduviidae: Triatominae) Tiago Belintani 1 , Jader Oliveira 2 , Heloisa Pinotti 3, Kaio Cesar Chaboli Alevi 3, Juliana Damieli Nascimento 1, Estela Sasso-Cerri 4, Cleber Galvão 5,* and João Aristeu da Rosa 3 1 Institute of Biology, Campinas State University (Unicamp), Block O, Bertrand Russel Avenue, Campinas 13083-865, São Paulo, Brazil; [email protected] (T.B.); [email protected] (J.D.N.) 2 Laboratory of Entomology in Public Health, Department of Epidemiology, Faculty of Public Health, University of São Paulo, Av. Arnaldo 01246-904, São Paulo, Brazil; [email protected] 3 School of Pharmaceutical Sciences, São Paulo State University (Unesp), Araraquara-Jaú Highway, km 1, Campos Ville, Araraquara 14800-903, São Paulo, Brazil; [email protected] (H.P.); [email protected] (K.C.C.A.); [email protected] (J.A.d.R.) 4 Department of Morphology, Genetics, Orthodontics and Pediatric Dentistry, Dental School, São Paulo State University (Unesp), Rua Humaitá, 1680, Araraquara 14801-903, São Paulo, Brazil; [email protected] 5 National and International Reference Laboratory in Taxonomy of Triatomines, Oswaldo Cruz Institute, Fiocruz, Av. Brasil, 4365, Manguinhos 21040-360, Rio de Janeiro, Brazil * Correspondence: [email protected] Citation: Belintani, T.; Oliveira, J.; Pinotti, H.; Alevi, K.C.C.; Nascimento, J.D.; Sasso-Cerri, E.; Galvão, C.; Simple Summary: We present a morphological and morphometric study with T. garciabesi, T. guasayana, Aristeu da Rosa, J. Characterization of T. patagonica, and T. sordida sensu stricto species within the Triatoma genus. -

Triatoma Melanica? Rita De Cássia Moreira De Souza1*†, Gabriel H Campolina-Silva1†, Claudia Mendonça Bezerra2, Liléia Diotaiuti1 and David E Gorla3
Souza et al. Parasites & Vectors (2015) 8:361 DOI 10.1186/s13071-015-0973-4 RESEARCH Open Access Does Triatoma brasiliensis occupy the same environmental niche space as Triatoma melanica? Rita de Cássia Moreira de Souza1*†, Gabriel H Campolina-Silva1†, Claudia Mendonça Bezerra2, Liléia Diotaiuti1 and David E Gorla3 Abstract Background: Triatomines (Hemiptera, Reduviidae) are vectors of Trypanosoma cruzi, the causative agent of Chagas disease, one of the most important vector-borne diseases in Latin America. This study compares the environmental niche spaces of Triatoma brasiliensis and Triatoma melanica using ecological niche modelling and reports findings on DNA barcoding and wing geometric morphometrics as tools for the identification of these species. Methods: We compared the geographic distribution of the species using generalized linear models fitted to elevation and current data on land surface temperature, vegetation cover and rainfall recorded by earth observation satellites for northeastern Brazil. Additionally, we evaluated nucleotide sequence data from the barcode region of the mitochondrial cytochrome c oxidase subunit I (CO1) and wing geometric morphometrics as taxonomic identification tools for T. brasiliensis and T. melanica. Results: The ecological niche models show that the environmental spaces currently occupied by T. brasiliensis and T. melanica are similar although not equivalent, and associated with the caatinga ecosystem. The CO1 sequence analyses based on pair wise genetic distance matrix calculated using Kimura 2-Parameter (K2P) evolutionary model, clearly separate the two species, supporting the barcoding gap. Wing size and shape analyses based on seven landmarks of 72 field specimens confirmed consistent differences between T. brasiliensis and T. melanica. Conclusion: Our results suggest that the separation of the two species should be attributed to a factor that does not include the current environmental conditions. -

High Triatoma Brasiliensis Densities and Trypanosoma
Am. J. Trop. Med. Hyg., 96(6), 2017, pp. 1456–1459 doi:10.4269/ajtmh.16-0823 Copyright © 2017 by The American Society of Tropical Medicine and Hygiene High Triatoma brasiliensis Densities and Trypanosoma cruzi Prevalence in Domestic and Peridomestic Habitats in the State of Rio Grande do Norte, Brazil: The Source for Chagas Disease Outbreaks? Mauricio Lilioso,1* Elaine Folly-Ramos,1 Fabiana Lopes Rocha,1 Jorge Rabinovich,2 Claire Capdevielle-Dulac,3 Myriam Harry,3 Paula L. Marcet,4 Jane Costa,5† and Carlos Eduardo Almeida1,6*† 1Programa de Pós-Graduação em Ecologia e Monitoramento Ambiental (PPGEMA), Universidade Federal da Paraíba (UFPB), Joao Pessoa, Brazil; 2Centro de Estudios Parasitológicos y de Vectores (CEPAVE), Universidad Nacional de La Plata, La Plata, Argentina; 3UMR Evolution, Génomes, Comportement, Ecologie, CNRS-IRD-Univ. Paris-Sud, Université Paris Saclay, Paris, France; 4Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention (CDC), Atlanta, Georgia; 5Laboratorio de Biodiversidade Entomologica, Instituto Oswaldo Cruz, FIOCRUZ, Rio de Janeiro, Brazil; 6Universidade Estadual de Campinas (UNICAMP), Campinas, Brazil Abstract. A total of 2,431 Triatoma brasiliensis were collected from 39 populations of Paraíba (PB) and Rio Grande do Norte (RN) states, Brazil. In PB, Trypanosoma cruzi infection was not detected in either peridomestic or domestic vector populations. In contrast, in RN, T. brasiliensis was detected with high parasite prevalence in these ecotopes (30.7–40.0%). Moreover, peridomicile insect population densities were more than double the average densities of all other settings evaluated (19.17 versus < 8.94 triatomine/man-hour). Genotyped parasites evidenced a mix of T. -
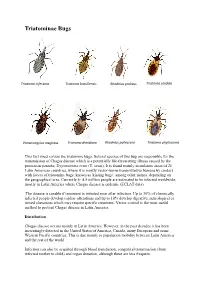
Triatominae Bugs
Triatominae Bugs Triatoma infestans Triatoma brasiliensis Rhodnius prolixus Triatoma sordida Panstrongylus megistus Triatoma dimidiata Rhodnius pallescens Triatoma phyllosoma This fact sheet covers the triatomine bugs. Several species of this bug are responsible for the transmission of Chagas disease which is a potentially life-threatening illness caused by the protozoan parasite, Trypanosoma cruzi (T. cruzi). It is found mainly in endemic areas of 21 Latin American countries, where it is mostly vector-borne transmitted to humans by contact with faeces of triatomine bugs, known as 'kissing bugs', among other names, depending on the geographical area. Currently 4- 4.5 million people are estimated to be infected worldwide, mostly in Latin America where Chagas disease is endemic (ECLAT data). The disease is curable if treatment is initiated soon after infection. Up to 30% of chronically infected people develop cardiac alterations and up to 10% develop digestive, neurological or mixed alterations which may require specific treatment. Vector control is the most useful method to prevent Chagas disease in Latin America. Distribution Chagas disease occurs mainly in Latin America. However, in the past decades it has been increasingly detected in the United States of America, Canada, many European and some Western Pacific countries. This is due mainly to population mobility between Latin America and the rest of the world. Infection can also be acquired through blood transfusion, congenital transmission (from infected mother to child) and organ donation, although these are less frequent. Transmission In Latin America, T. cruzi parasites are mainly transmitted by contact with the faeces of infected blood-sucking triatomine bugs. These bugs, vectors that carry the parasites, typically live in the cracks of poorly-constructed homes in rural or suburban areas.