Start-Et-Al-2018-Evolution.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cities Serve As Testbeds for Evolutionary Change NEWS FEATURE Urban Living Can Pressure Flora and Fauna to Adapt in Intriguing Ways
NEWS FEATURE Cities serve as testbeds for evolutionary change NEWS FEATURE Urban living can pressure flora and fauna to adapt in intriguing ways. Biologists are starting to take advantage of this convenient laboratory of evolution. Carolyn Beans, Science Writer Every student of evolution knows the story of the peppered moth. The species comes in two colors: one a peppered white, the other black. During Britain’s industrial revolution, hungry birds spotted the lighter morph in soot-coated forests surrounding cities. Meanwhile, the rarer and better camouflaged darker morph avoided becoming lunch and carried the darker gene variant to a higher frequency in the pop- ulation. When pollution cleared, the lighter morph again became more common. Although the method- ology of the original peppered moth research came into question in the late 1990s (1), subsequent re- search confirmed its findings (2). Yet despite this classic case of natural selection under urban conditions, so iconic that the peppered moth adorns the logo of the Society for the Study of Evolution, biologists have mainly chosen to study evolution in places with less human disturbance. “Most people didn’t think that cities were really inter- esting biologically, that they were kind of anti-life,” Researchers are studying white clover in cities across the globe to learn more says evolutionary ecologist Marc Johnson, who directs about how urbanization shapes evolutionary change. Image credit: Marc the Centre for Urban Environments at the University of Johnson (University of Toronto Mississauga, Mississauga, Canada). Toronto Mississauga. That mindset is changing as Johnson and others to navigate Puerto Rican cities, an ant gained heat use cities as powerful testbeds for evolutionary mech- tolerance in the urban heat island of Cleveland, and anisms. -

Azerbaijani Millionaire Haji Zeynalabdin Taghiyev's Attitude Towards Cultural Heritage
YOUTH IN CONSERVATION OF CULTURAL HERITAGE – YOCOCU 2016, MADRID- BOOK OF ABSTRACTS 1 YOUTH IN CONSERVATION OF CULTURAL HERITAGE – YOCOCU 2016, MADRID- BOOK OF ABSTRACTS 2 Coordinated by: Instituto de Geociencias IGEO (CSIC, UCM) Co-organized by: Youth in Conservation of Cultural Heritage - YOCOCU España Museo Centro de Arte Reina Sofía MNCARS Fundación Reina Sofía Edited by: Mónica Álvarez de Buergo Beatriz Cámara Gallego Duygu Ergenc Sofía Melero Tur Elena Mercedes Pérez-Monserrat First edition Madrid © editorial work, the editors © texts, their authors © images, their authors © YOCOCU 2016 logo, Laura López © front cover image, Sofía Melero Tur ISBN: 978-84-617-4237-0 YOUTH IN CONSERVATION OF CULTURAL HERITAGE – YOCOCU 2016, MADRID- BOOK OF ABSTRACTS SCIENTIFIC COMMITTEE Elia María ALONSO GUZMÁN Universidad Michoacana de San Nicolás de Hidalgo, Mexico Francisco Javier ALONSO University of Oviedo, Spain Nevin ALY Suez University, Egypt Joaquín BARRIO Universidad Autónoma de Madrid, Spain Majda BEGIC Croatian Conservation Institute, YOCOCU Croatia David BENAVENTE Universidad de Alicante, Spain Manuel BETHENCOURT Universidad de Cádiz, Spain María Teresa BLANCO Instituto Eduardo Torroja de la Construcción y del Cemento, CSIC, Madrid, Spain Ernesto BORRELLI Istituto Superiore per la Conservazione ed il Restauro, Italy Maria BRAI University of Palermo, Italy Maarten A.T.M. BROEKMANS Geological Survey of Norway - NGU Simone CAGNO Belgian Nuclear Research Center, YOCOCU Belgium Ana CALVO Spanish Group International Institute for Conservation -

Artificial Light at Night As a Driver of Evolution Across Urban–Rural Landscapes
CONCEPTS AND QUESTIONS 1 Artificial light at night as a driver of evolution across urban–rural landscapes Gareth R Hopkins1*, Kevin J Gaston2, Marcel E Visser3, Mark A Elgar1, and Therésa M Jones1 Light is fundamental to biological systems, affecting the daily rhythms of bacteria, plants, and animals. Artificial light at night (ALAN), a ubiquitous feature of urbanization, interferes with these rhythms and has the potential to exert strong selection pres- sures on organisms living in urban environments. ALAN also fragments landscapes, altering the movement of animals into and out of artificially lit habitats. Although research has documented phenotypic and genetic differentiation between urban and rural organisms, ALAN has rarely been considered as a driver of evolution. We argue that the fundamental importance of light to bio- logical systems, and the capacity for ALAN to influence multiple processes contributing to evolution, makes this an important driver of evolutionary change, one with the potential to explain broad patterns of population differentiation across urban–rural landscapes. Integrating ALAN’s evolutionary potential into urban ecology is a targeted and powerful approach to understanding the capacity for life to adapt to an increasingly urbanized world. Front Ecol Environ 2018; 16(8): 1–8, doi:10.1002/fee.1828 ight is fundamental to life on Earth. One constancy in the documented in all higher taxonomic groups (Tan et al. 2010) is L evolution of life has been the roughly 24- hour oscillation a key regulator of these biological rhythms, and melatonin is a between a bright day, with a light intensity of around 1000– powerful antioxidant with important fitness effects (Tan et al. -

An Introgression Analysis of Quantitative Trait Loci That Contribute to a Morphological Difference Between Drosophila Simulans and D
Copyight 0 1997 by the Genetics Societv of America An Introgression Analysis of Quantitative Trait Loci That Contribute to a Morphological Difference Between Drosophila simulans and D. mauritiana Cathy C. Laurie, John R. True, Jianjun Liu and John M. Mercer Department of Zoology, Duke University, Durham, North Carolina 27708 Manuscript received August 12, 1996 Accepted for publication October 29, 1996 ABSTRACT Drosophila simulans and D. maum'tiana differ markedly in morphology of the posterior lobe, a male- specific genitalic structure. Bothsize and shapeof the lobe can be quantifiedby a morphometric variable, PC1, derived from principal components and Fourier analyses. The genetic architectureof the species difference in PC1 was investigated previously by composite interval mapping, which revealed largely additive inheritance, with a minimum of eight quantitative trait loci (QTL) affecting the trait. This analysis was extended by introgression of marked segments of the maun'tiana third chromosome into a simulans background by repeated backcrossing. Thetwo types of experiment are consistentin suggesting that several QTL on the third chromosome may have effects in the range of 10-15% of the parental difference andthat all or nearly all QTL have effects in the same direction. Since the parental difference is large (30.4 environmental standard deviations), effects of this magnitude can produce alternative homozygotes with little overlap in phenotype. However, these estimates may not reflect the effects of individual loci, since each interval or introgressed segment may contain multiple QTL. The consistent direction of allelic effects suggests a history of directional selection on the posterior lobe. HE genetic analysis of a quantitative trait usually ual genes that affect continuously variable traits. -

Microevolution and the Genetics of Populations Microevolution Refers to Varieties Within a Given Type
Chapter 8: Evolution Lesson 8.3: Microevolution and the Genetics of Populations Microevolution refers to varieties within a given type. Change happens within a group, but the descendant is clearly of the same type as the ancestor. This might better be called variation, or adaptation, but the changes are "horizontal" in effect, not "vertical." Such changes might be accomplished by "natural selection," in which a trait within the present variety is selected as the best for a given set of conditions, or accomplished by "artificial selection," such as when dog breeders produce a new breed of dog. Lesson Objectives ● Distinguish what is microevolution and how it affects changes in populations. ● Define gene pool, and explain how to calculate allele frequencies. ● State the Hardy-Weinberg theorem ● Identify the five forces of evolution. Vocabulary ● adaptive radiation ● gene pool ● migration ● allele frequency ● genetic drift ● mutation ● artificial selection ● Hardy-Weinberg theorem ● natural selection ● directional selection ● macroevolution ● population genetics ● disruptive selection ● microevolution ● stabilizing selection ● gene flow Introduction Darwin knew that heritable variations are needed for evolution to occur. However, he knew nothing about Mendel’s laws of genetics. Mendel’s laws were rediscovered in the early 1900s. Only then could scientists fully understand the process of evolution. Microevolution is how individual traits within a population change over time. In order for a population to change, some things must be assumed to be true. In other words, there must be some sort of process happening that causes microevolution. The five ways alleles within a population change over time are natural selection, migration (gene flow), mating, mutations, or genetic drift. -

Patterns and Power of Phenotypic Selection in Nature
Articles Patterns and Power of Phenotypic Selection in Nature JOEL G. KINGSOLVER AND DAVID W. PFENNIG Phenotypic selection occurs when individuals with certain characteristics produce more surviving offspring than individuals with other characteristics. Although selection is regarded as the chief engine of evolutionary change, scientists have only recently begun to measure its action in the wild. These studies raise numerous questions: How strong is selection, and do different types of traits experience different patterns of selection? Is selection on traits that affect mating success as strong as selection on traits that affect survival? Does selection tend to favor larger body size, and, if so, what are its consequences? We explore these questions and discuss the pitfalls and future prospects of measuring selection in natural populations. Keywords: adaptive landscape, Cope’s rule, natural selection, rapid evolution, sexual selection henotypic selection occurs when individuals with selection on traits that affect survival stronger than on those Pdifferent characteristics (i.e., different phenotypes) that affect only mating success? In this article, we explore these differ in their survival, fecundity, or mating success. The idea and other questions about the patterns and power of phe- of phenotypic selection traces back to Darwin and Wallace notypic selection in nature. (1858), and selection is widely accepted as the primary cause of adaptive evolution within natural populations.Yet Darwin What is selection, and how does it work? never attempted to measure selection in nature, and in the Selection is the nonrandom differential survival or repro- century following the publication of On the Origin of Species duction of phenotypically different individuals. -

Gridlock and Beltways: the Genetic Context of Urban Invasions
Oecologia (2020) 192:615–628 https://doi.org/10.1007/s00442-020-04614-y CONCEPTS, REVIEWS AND SYNTHESES Gridlock and beltways: the genetic context of urban invasions E. M. X. Reed1 · M. E. Serr1 · A. S. Maurer1 · M. O. Burford Reiskind1 Received: 8 May 2019 / Accepted: 30 January 2020 / Published online: 13 February 2020 © Springer-Verlag GmbH Germany, part of Springer Nature 2020 Abstract The rapid expansion of urban land across the globe presents new and numerous opportunities for invasive species to spread and fourish. Ecologists historically rejected urban ecosystems as important environments for ecology and evolution research but are beginning to recognize the importance of these systems in shaping the biology of invasion. Urbanization can aid the introduction, establishment, and spread of invaders, and these processes have substantial consequences on native species and ecosystems. Therefore, it is valuable to understand how urban areas infuence populations at all stages in the invasion process. Population genetic tools are essential to explore the driving forces of invasive species dispersal, connectivity, and adaptation within cities. In this review, we synthesize current research about the infuence of urban landscapes on invasion genetics dynamics. We conclude that urban areas are not only points of entry for many invasive species, they also facilitate population establishment, are pools for genetic diversity, and provide corridors for further spread both within and out of cities. We recommend the continued use of genetic studies to inform invasive species management and to understand the underlying ecological and evolutionary processes governing successful invasion. Keywords Synthesis · Population genetics · Landscape genetics · Invasive species · Urban ecosystems Introduction increasing extent of urban land cover and the synergistic relationship between urbanization and invasion (Seto et al. -

Urban Evolution: the Role of Water
Water 2015, 7, 4063-4087; doi:10.3390/w7084063 OPEN ACCESS water ISSN 2073-4441 www.mdpi.com/journal/water Review Urban Evolution: The Role of Water Sujay S. Kaushal 1,*, William H. McDowell 2, Wilfred M. Wollheim 2, Tamara A. Newcomer Johnson 1, Paul M. Mayer 3, Kenneth T. Belt 4 and Michael J. Pennino 5 1 Department of Geology & Earth System Science Interdisciplinary Center, University of Maryland, College Park, MD 21201, USA; E-Mail: [email protected] 2 Department of Natural Resources and the Environment, University of New Hampshire, Durham, NH 03824, USA; E-Mails: [email protected] (W.H.M.); [email protected] (W.M.W.) 3 US Environmental Protection Agency, National Health and Environmental Effects Research Lab, Western Ecology Division, Corvallis, OR 97333, USA; E-Mail: [email protected] 4 United States Department of Agriculture Forest Service, Northern Research Station, Baltimore, MD 21228, USA; E-Mail: [email protected] 5 Department of Civil and Environmental Engineering, Princeton University, Princeton, NJ 08544, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-301-405-7048. Academic Editor: Fabio Masi Received: 21 April 2015 / Accepted: 17 July 2015 / Published: 27 July 2015 Abstract: The structure, function, and services of urban ecosystems evolve over time scales from seconds to centuries as Earth’s population grows, infrastructure ages, and sociopolitical values alter them. In order to systematically study changes over time, the concept of “urban evolution” was proposed. It allows urban planning, management, and restoration to move beyond reactive management to predictive management based on past observations of consistent patterns. -
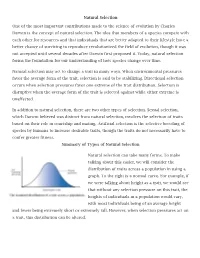
Natural Selection One of the Most Important Contributions Made to The
Natural Selection One of the most important contributions made to the science of evolution by Charles Darwin is the concept of natural selection. The idea that members of a species compete with each other for resources and that individuals that are better adapted to their lifestyle have a better chance of surviving to reproduce revolutionized the field of evolution, though it was not accepted until several decades after Darwin first proposed it. Today, natural selection forms the foundation for our understanding of how species change over time. Natural selection may act to change a trait in many ways. When environmental pressures favor the average form of the trait, selection is said to be stabilizing. Directional selection occurs when selection pressures favor one extreme of the trait distribution. Selection is disruptive when the average form of the trait is selected against while either extreme is unaffected. In addition to natural selection, there are two other types of selection. Sexual selection, which Darwin believed was distinct from natural selection, involves the selection of traits based on their role in courtship and mating. Artificial selection is the selective breeding of species by humans to increase desirable traits, though the traits do not necessarily have to confer greater fitness. Summary of Types of Natural Selection Natural selection can take many forms. To make talking about this easier, we will consider the distribution of traits across a population in using a graph. To the right is a normal curve. For example, if we were talking about height as a trait, we would see that without any selection pressure on this trait, the heights of individuals in a population would vary, with most individuals being of an average height and fewer being extremely short or extremely tall. -

Supplementary Information for Vyssotski, 2013
Supplementary Information for Vyssotski, 2013 www.evolocus.com/evolocus/v1/evolocus-01-013-s.pdf Evolocus Supplementary Information for Transgenerational epigenetic compensation and sex u al d imorph ism 1,2,3 Dmitri L. Vyssotski Published 17 May 2013, Evolocus 1, 13-18 (2013): http://www.evolocus.com/evolocus/v1/evolocus-01-013.pdf In natu ral popu lation transgenerational epigenetic compensation is by unknown mechanisms, the mechanisms those were difficult to generated in h omoz y gou s mu tants, mainly in males. It d oes not imagine, and this possibility was abandoned for over 47 years. immed iately affect th e ph enoty pe of h omoz y gou s mu tants, w h ere The higher variability in males can be a result of a not so good it w as generated . Transgenerational epigenetic compensation is canalization of their ontogenesis, and the canalization of localiz ed not in th e same locu s as original mu tation and it is ontogenesis is modulated by sex hormones. Thus, we can d ominant, b ecau se it affects th e ph enoty pe ev en if it is receiv ed imagine that the canalization of ontogenesis in males is from one parent. In fu rth er generations, starting from F 2, it is more artificially decreased in comparison with females by means of ex pressed in females th an in males: it increases fitness of hormonal differences. h omozy gou s mu tant females, d ecreases fitness of w ild ty pe The shift of the female distribution to the right was not females and h as w eak effect on h eterozy gou s females. -

Stabilizing Selection Shapes Variation in Phenotypic Plasticity
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.29.454146; this version posted July 29, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Stabilizing selection shapes variation in phenotypic plasticity 2 3 4 5 Dörthe Becker1,2,*, 6 Karen Barnard-Kubow1,3, 7 Robert Porter1, 8 Austin Edwards1,4, 9 Erin Voss1,5, 10 Andrew P. Beckerman2, 11 Alan O. Bergland1,* 12 13 14 1Department of Biology; University of Virginia, Charlottesville/VA, USA 15 2Department of Animal and Plant Sciences; University of Sheffield, Sheffield, UK 16 3Department of Biology, James Madison University, Harrisonburg/VA, USA 17 4Biological Imaging Development CoLab, University of California San Francisco, 18 San Francisco/CA, USA 19 5Department of Integrative Biology, University of California Berkeley, 20 Berkeley/CA 21 22 * to whom correspondence should be addressed: 23 DB ([email protected]), AOB ([email protected]) 24 25 26 27 Keywords: stabilizing selection; phenotypic plasticity; heritability; Daphnia; 28 defense morphologies 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.07.29.454146; this version posted July 29, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 29 Abstract 30 The adaptive nature of phenotypic plasticity is widely documented in natural 31 populations. However, little is known about the evolutionary forces that shape 32 genetic variation in plasticity within populations. Here we empirically address this 33 issue by testing the hypothesis that stabilizing selection shapes genetic variation 34 in the anti-predator developmental plasticity of Daphnia pulex. -

Selection on Breeding Date and Body Size in Colonizing Coho Salmon, Oncorhynchus Kisutch
Molecular Ecology (2010) 19, 2562–2573 doi: 10.1111/j.1365-294X.2010.04652.x Selection on breeding date and body size in colonizing coho salmon, Oncorhynchus kisutch J. H. ANDERSON,* P. L. FAULDS,† W. I. ATLAS,‡ G. R. PESS§ and T. P. QUINN* *School of Aquatic and Fishery Sciences, University of Washington, Box 355020, Seattle, WA 98195, USA, †Seattle Public Utilities, 700 5th Avenue, Suite 4900, Seattle, WA, 98124, USA, ‡Department of Biological Sciences, Simon Fraser University, 8888 University Drive, Burnaby, BC, Canada V5A 1S6, §National Marine Fisheries Service, Northwest Fisheries Science Center, 2725 Montlake Boulevard East, Seattle, WA 98195, USA Abstract Selection during the colonization of new habitat is critical to the process of local adaptation, but has rarely been studied. We measured the form, direction, and strength of selection on body size and date of arrival to the breeding grounds over the first three cohorts (2003–2005) of a coho salmon (Oncorhynchus kisutch) population colonizing 33 km of habitat made accessible by modification of Landsburg Diversion Dam, on the Cedar River, Washington, USA. Salmon were sampled as they bypassed the dam, parentage was assigned based on genotypes from 10 microsatellite loci, and standardized selection gradients were calculated using the number of returning adult offspring as the fitness metric. Larger fish in both sexes produced more adult offspring, and the magnitude of the effect increased in subsequent years for males, suggesting that low densities attenuated traditional size-biased intrasexual competition. For both sexes, directional selection favoured early breeders in 2003, but stabilizing selection on breeding date was observed in 2004 and 2005.