Anaerobic Soil Disinfestation: Meta-Analysis and Optimization of Amendment Carbon Rate and C:N Ratio to Control Key Plant Pathogens and Weeds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

First Record of the Sedge Feeder Bactra Verutana Zeller (Lepidoptera
Revista Brasileira de Entomologia 63 (2019) 104–107 REVISTA BRASILEIRA DE Entomologia A Journal on Insect Diversity and Evolution www.rbentomologia.com Short Communication First record of the sedge feeder Bactra verutana Zeller (Lepidoptera: Tortricidae) in Chile based on morphology and DNA barcodes a,∗ b Héctor A. Vargas , Marcelo Vargas-Ortiz a Universidad de Tarapacá, Facultad de Ciencias Agronómicas, Departamento de Recursos Ambientales, Arica, Chile b Universidad de Concepción, Facultad de Ciencias Naturales y Oceanográficas, Departamento de Zoología, Programa de Doctorado en Sistemática y Biodiversidad Concepción, Chile a r a b s t r a c t t i c l e i n f o Article history: The sedge-feeding moth Bactra verutana Zeller, 1875 (Lepidoptera: Tortricidae: Olethreutinae: Bactrini), Received 4 October 2018 described from Dallas, Texas, USA, is widespread, recorded throughout much North America, Central Accepted 27 February 2019 and South America, including the Caribbean, and Africa. The species is recorded for the first time from Available online 21 March 2019 Chile based on specimens collected in the coastal valleys of the Atacama Desert, where its larvae feed Associate Editor: Livia Pinheiro on Cyperus corymbosus Rottb. var. subnodosus (Nees & Meyen) Kük. (Cyperaceae). A single DNA barcode haplotype, which is widespread in USA, was found in two Chilean specimens sequenced. Keywords: © 2019 Sociedade Brasileira de Entomologia. Published by Elsevier Editora Ltda. This is an open Atacama Desert Cyperaceae access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). Cyperus corymbosus DNA barcoding Bactra Stephens, 1834 (Olethreutinae: Bactrini) is a widespread sequences (sensu Hebert et al., 2003) were used to assess the rela- genus of Tortricidae (Lepidoptera) with 106 described species tionships of the Chilean specimens. -

Bulletin 132
fj/^ (U^^tj^.J-'^'^^^^ ^^^^ S>nTHSONI.\N INSTITTTION UNITED STATES NATIONAL MUSEUM Bulletin 132 REVISION OF THE NORTH AMERICAN MOTHS OF THE SUBFAMILIES LASPEYRESIINAE AND OLETHREUTINAE BY CARL HEINRICH Of the Bureau of Entomology, United St^es Deparimirtt of Agriculture WASHINGTON GOVERNMENT PRINTING OFFICE 1926 ADDITIONAL COPIES OF THIS PUBLICATION MAY BE PROCURED FROM THE SUPERINTENDENT OF DOCUMENTS GOVERNMENT PRINTING OFFICE WASHINGTON, D. C. AT 75 CENTS PER COPY — ADVERTISEMENT The scientific publications of the National Museum consist of two series Proceedings and Bulletins. The Proceedings, the first volume of which was issued in 1878, are intended primarily as a medium for the publication of original papers based on the collections of the National Museum, setting forth newly acquired facts in biology, anthropology, and geology derived therefrom, or containing descriptions of new forms and revisions of limited groups. One or two volumes are completed annually and copies of each paper, in pamphlet form, are dis- tributed as soon as published to libraries and scientific organizations and to specialists and others interested in the different subjects. The dates at which these separate papers are published are recorded in the table of contents of the volume. The Bulletins^ the first of which was issued in 1875, consist of a series of separate organizations comprising chiefly monographs of large zoological groups and other general systematic treatises (occa- sionally in several volumes), faunal works, reports of expeditions, and catalogues of type specimens, special collections, etc. The majority of the volumes are octavos, but a quarto size has been adopted in a few instances in which large plates were regarded as indispensable. -

Effect of Seed's Age on Some Treatments' Efficiency for Breaking
International Journal of Scientific and Research Publications, Volume 10, Issue 8, August 2020 633 ISSN 2250-3153 Effect of Herbicides on Living Organisms in The Ecosystem and Available Alternative Control Methods. USTUNER T *, AL SAKRAN M*, ALMHEMED K*, * Department of Plant Protection, Faculty of Agriculture, Kahramanmaras Sutcu Imam University, Kahramanmaras-Turkey. DOI: 10.29322/IJSRP.10.08.2020.p10480 http://dx.doi.org/10.29322/IJSRP.10.08.2020.p10480 Corresponding author: [email protected] Abstract- Herbicides are used in agricultural areas to reduce chemical structure. In Turkey, the aim of herbicides using is not harmful of weeds. Herbicides could decimate some weeds and to completely destroy weeds, but to reduce the weeds slow down growth in others. Using herbicide has increased population and to prevent it to compete with crops (Kraehmer, significantly since the mid-20th century. In case that herbicides 2012; Güncan, 2013). In a 20-year study conducted in are not used, significant quantitative and qualitative losses will Denmark, it was reported that 80% of 200 weed species grown have occurred in agricultural production. However, the side in the cultivated fields are too weak to compete with the crops effects caused by the wide and irrational use of herbicides and therefore do not affect the yield (Andreasen et al., 1996). In threaten the environment and human health. Although addition, weeds contribute positively to the ecosystem by herbicides are the least harmful among pesticides, many studies contributing to biodiversity. Weeds prevent soil damage have shown the serious negative effects of herbicides on the through erosion that may occur after harvest. -

Lepidoptera (Moths and Butterflies) at Inverness Ridge in Central Coastal California and Their Recovery Following a Wildfire
LEPIDOPTERA (MOTHS AND BUTTERFLIES) AT INVERNESS RIDGE IN CENTRAL COASTAL CALIFORNIA AND THEIR RECOVERY FOLLOWING A WILDFIRE J. A. Powell Essig Museum of Entomology, University of California, Berkeley, CA 94720 Abstract.— In numbers of species, Lepidoptera (butterflies and moths) make up the largest group of plant-feeding animals in North America. Caterpillars of nearly all species feed on plants, and most of them are specialists on one or a few kinds of plants. Therefore they are liable to be severely affected by wildfires, and secondarily, their parasites and predators, including birds, bats, lizards, and rodents, suffer losses of a major food resource. In October 1995, a wildfire swept over part of The Point Reyes National Seashore, burning more than 12,300 acres (5,000 hectares) of public and private land, following a fire-free period of several decades. I tracked survival and recolonization by moths and butterflies during the subsequent five seasons. I made daytime searches for adults and caterpillars approximately monthly from March through October and collected blacklight trap samples, mostly in May and September-October. More than 650 species of Lepidoptera have been recorded in the Inverness Ridge area, and about 375 of them were recorded during the post-fire survey, including larvae of 31% of them. Plants in a Bishop pine forest higher on the ridge, where the fire was most intense, accumulated their caterpillar faunas slowly, while Lepidoptera feeding on plants typical of riparian woods in the lower canyons reestablished sooner and more completely. Recolonization varied markedly among different plant species, and the species richness gradually increased, in marked contrast to generalizations about effects of fire on arthropods derived from fire management of grasslands. -

Mcqs of Weed Management
MCQs of Weed Management Department of Agronomy College of Agriculture, NAU, Bharuch Q.1. Commelina benghalensis bearing short- of lived blue coloured flowers is a a). Free floating a). Monocot b). Rooted floating b). Dicot c). Rooted submerged c). Spermatophyte d). Emergent Q.11. The partial root parasite d). Pteridophyta a). Cuscuta Q.2. The most systematic method for classify- b). Loranthus ing weeds is based on c). Striga a). Morphology d). Orobanche b). Life history Q.12. A weed with a funnel shaped corolla c). Habitat a). Medicago denticulata d). Phylogenetic b). Vicia sativa Q.3. Simple perennials are reproduced by c). Convolvulus arvensis a). Rhizomes d). Scirpus sp b). Tubers Q.13. A sedge with rhizomes c). Corms a). Commelina obliqua d). Seeds b). Cyperus rotundus Q.4. The first prominent instance of biochemi- c). Scirpus sp cal mimicry based crop associated weed d). Eleocharis under Indian perspective is Q.14. A lowland rice sedge a). Saccharum spontaneous in sugarcane a). Cyperus iria b). Phalaris minor in wheat b). Cyperus difformis c). Wild rice (Oryza longistaninata) in rice c). Cyperus esculentus d). Itch grass (Rottboellea cochinchinen- d). All sis) in upland rice Q.15. The ‘condition influences’ directly plant Q.5. A weed with a trailing stem ability in exploring resources a).Convolvulus arvensis a). Soil density b). Digitaria sanguinalis b). Soil CO2 c). Cuscuta sp c). Soil N d). Cynodon sp d). Soil water Q.6. A weed with a balloon structure for effec- Q.16. Hans Molish is associated with tive dissemination a). Competition a). Physalis minima b). -

RAZOWSKI J., BECKER V. O. Systematic and Faunistic Data On
POLISH JOURNAL OF ENTOMOLOGY POLSKIE P I S M O ENTOMOLOGICZNE VOL. 79 : 187-193 Bydgoszcz 30 June 2010 Systematic and faunistic data on Neotropical Bactrini (Lepidoptera: Tortricidae) JÓZEF RAZOWSKI *, V ITOR O. B ECKER ** * Institute of Systematics and Evolution of Animals PAS, Sławkowska 17, 31-016 Kraków, Poland, e-mail: [email protected] ** Reserve Serra Bonita, PO Box 01, 45880-970 Camacan, BA, Brazil ABSTRACT. Neotropical species of Bactra and Endothenia are listed and commented. Seven species are recorded from Central and South America. Bactra goiasia and Endothenia tuxtlasia are described as new. KEY WORDS: Lepidoptera, Tortricidae, new species, new data, Neotropic. INTRODUCTION A. DIAKONOFF the late leading specialist to Bactra and its allies dealt (DIAKONOFF 1964) with ten species of this genus found in the Neotropics. Currently 13 species (see the list below) are recorded. This cosmopolitan genus is widely destributed and known from all geographic regions. Another genus, Endothenia currently included in this tribe is represented by three Neotropi- cal species. The Holarctic E. hebesana (WALKER , 1863) was mentioned from Puerto Rico but this identification should be confirmed. Some data on Neotropical Endothenia (included in Endotheniina) are provided by RAZOWSKI & PELZ (2002). 188 Polish Journal of Entomology 79 (2) Systematic list of Neotropical Bactrini Bactra STEPHENS , 1834 goiasia sp. n., Brazil: Goias; male genitalia; this paper. diachorda M EYRICK , 1932 ( Bactra ), Brazil: Santa Catarina; D IAKONOFF (1964): male genitalia. seria M EYRICK , 1917 ( Bactra ), Peru; DIAKONOFF (1964): male and female genitalia. clarkei D IAKONOFF , 1964 ( Bactra ), British Guiana: male and female genitalia. adoceta D IAKONOFF , 1964 ( Bactra ); Brazil: Paraná; male genitalia. -

A Preliminary List of the Leaf-Roller Moths (Lepidoptera: Tortricidae) of Virginia
Banisteria, Number 38, pages 3-37 © 2011 Virginia Natural History Society A Preliminary List of the Leaf-roller Moths (Lepidoptera: Tortricidae) of Virginia Winnie H.Y. Lam Department of Biological Sciences CW 405 Biological Sciences Building University of Alberta Edmonton, Alberta, Canada T6G 2E9 (email: [email protected]) Jadranka Rota1 Department of Entomology National Museum of Natural History Smithsonian Institution Washington, DC 20013-7012, USA John W. Brown2 Systematic Entomology Laboratory U.S. Department of Agriculture, A.R.S. National Museum of Natural History P.O. Box 37012, MRC 168 Washington, DC 20013-7012, USA (email: [email protected]) ABSTRACT The microlepidopteran fauna of Virginia is poorly documented. We present an annotated checklist of 301 species of leaf-roller moths (Lepidoptera: Tortricidae) recorded from the state based on the examination of 4,207 pinned specimens deposited in institutional or university collections; the specimen database from the Essig Museum of Entomology, University of California, Berkeley (122 specimen records); and literature records. County distribution, capture dates, and host plants are presented for each species. The geographic coverage of the material examined is highly uneven, with most specimens (60%) from Fairfax County (200 species). The poor state of knowledge of the Virginia tortricid fauna is demonstrated by the lack of records for nearly one-fifth of all counties and large independent cities. Much more collecting by both amateur and professional lepidopterists, as well as a review of additional existing collections, is needed before a general understanding of the geographic and temporal distribution of Virginia’s tortricid fauna will begin to emerge. -

Crocidosema Aporema
CAPS Datasheets provide pest-specific information to support planning and completing early detection surveys. Crocidosema aporema a b Figure 1. Adult (a) male and (b) female Crocidosema aporema. Photos: Christi Jaeger, MEM. Scientific Name Crocidosema aporema (Walsingham, 1914) Synonym(s): Epinotia aporema (Walsingham, 1914) Epinotia opposita Heinrich, 1931 Eucosma aporema Walsingham, 1914 Common Name(s) Bud borer, bean shoot moth, soybean bud borer Type of Pest Moth, borer Taxonomic position: Class: Insecta, Order: Lepidoptera, Family: Tortricidae Reason for Inclusion in Manual: CAPS Soybean Commodity Survey list 2015-present Version 2 April 22, 2020 1 Pest Recognition This section describes characteristics of the pest and symptoms that may help surveyors recognize possible infestations in the field and select survey sites. For morphological descriptions, see Identification Resources on the AMPS pest page on the CAPS Resource and Collaboration website. Crocidosema aporema produce multiple, overlapping generations each year. Adults appear first but Figure 2. First (bottom) and third (top) instar Crocidosema larvae and adults may be active at aporema. Photo: Laboratório de Controle Integrado de Insetos the same time throughout the (http://www.bio.ufpr.br/portal/insectbiocontrol/). growing season (Ferreira, 1980; Altesor et al., 2010). Adult moths are small and brown. The adult wingspan is 14 to 17mm (approx. 0.55 to 0.67 in) wide (Morey, 1972; Ferreira, 1980) (Figs. 1a and b). Adults are nocturnal (Liljesthröm et al., 2001). During the day, adults rest within the vegetation, but flush and fly in short bursts if disturbed (Cano Ortiz, 1998; Wille, 1952). Daytime observations Figure 3. Late instar larva of Crocidosema aporema. -

Organic Farming S S Rana
Organic Farming S S Rana Department of Agronomy, COA, CSK HPKV, Palampur-176062 Organic Farming S S Rana Principal Scientist Department of Agronomy, College of Agriculture, CSK Himachal Pradesh Krishi Vishvavidyalaya, Palampur-176062 Copyright 2011 SS Rana , Principal Scientist, Department of Agronomy, Coll ege of Agriculture, CSK Himachal Pradesh Krishi Vishvavidyalaya, Palampur- 176062. No part of this pub lication may be reproduced, stored in a retrieval system, or transmitt ed, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise without the writt en permiss ion of Dr. SS Rana . Citation Rana SS 2011. Organic Farming . Department of Agronomy, College of Agriculture, CSK Himachal Pradesh Krishi Vishvavidyalaya, Palampur , pages. 90p Revised: 2016 PPPrrreeefffaaaccceee The prosperity of any country depends upon the prosperity of its people. Human race depends more on farm products for their existence than anything else since food and clothing – the prime necessaries are products of farming . Even for industrial prosperity, farming forms the basic raw material. The farming being practiced for the last five decades in India has increasingly been found non-sustainable. The system is oriented towards high production without much concern for ecology and the very existence of man himself. Adverse effects of modern agricultural practices not only on the farm but also on the health of all living things and thus on the environment have been well documented all over the world. The present system of agriculture which we call 'conventional' and practiced the world over is evolved in the western nations as a product of their socio- economic environment which promoted an over riding quest for accumulation of wealth. -
The Species Richness of Lepidoptera in a Fragmented Landscape: a Supplement to the Checklist of Moths of Butler County, Ohio
The Great Lakes Entomologist Volume 34 Number 1 - Spring/Summer 2001 Number 1 - Article 12 Spring/Summer 2001 April 2001 The Species Richness of Lepidoptera in a Fragmented Landscape: A Supplement to the Checklist of Moths of Butler County, Ohio Keith S. Summerville Miami University Thomas O. Crist Miami University Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Summerville, Keith S. and Crist, Thomas O. 2001. "The Species Richness of Lepidoptera in a Fragmented Landscape: A Supplement to the Checklist of Moths of Butler County, Ohio," The Great Lakes Entomologist, vol 34 (1) Available at: https://scholar.valpo.edu/tgle/vol34/iss1/12 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Summerville and Crist: The Species Richness of Lepidoptera in a Fragmented Landscape: A 2001 THE GREAT LAKES ENTOMOLOGIST 93 THE SPECIES RICHNESS OF lEPIDOPTERA IN A FRAGMENTED LANDSCAPE: A SUPPLEMENT TO THE CHECKLIST OF MOTHS OF BUTlER COUNTY, OHIO Keith S. Summerville 1 and Thomas O. Crist1 ABSTRACT Land conversion for agriculture or urban expansion has fragmented the midwestern landscape and isolated native biotas in remnant habitat patches. Identification of priority renmants to be targeted for conservation, however, requires an understanding of the species diversity and distributions in such fragmented landscapes. During a 3-year inventory, we estimated the species richness of Lepidoptera in forests and old fields within an agricultural region of southwest Ohio, Butler County. -

UC Riverside UC Riverside Electronic Theses and Dissertations
UC Riverside UC Riverside Electronic Theses and Dissertations Title Outcomes of Chronic Arsenic Exposure on Aquatic Insects Permalink https://escholarship.org/uc/item/9g9326qj Author Mogren, Christina Loraine Publication Date 2013 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA RIVERSIDE Outcomes of Chronic Arsenic Exposure on Aquatic Insects A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Entomology by Christina Loraine Mogren June 2013 Dissertation Committee: Dr. John T. Trumble, Chairperson Dr. Ring T. Cardé Dr. David R. Parker Dr. William E. Walton Copyright by Christina Loraine Mogren 2013 The Dissertation of Christina Loraine Mogren is approved: Committee Chairperson University of California, Riverside Acknowledgements I would like to first and foremost thank my major advisor, Dr. John Trumble, for all of his help and guidance throughout my graduate career, and particularly for the many opportunities to travel and present my research at professional meetings in this country and abroad. I would like to thank my dissertation committee members, Drs. William Walton, David Parker, and Ring Cardé, who were wonderfully understanding and helpful in providing guidance on their various areas of expertise that were critical to the success of this research. I would also like to thank Drs. Brad Mullens and Joao Pedra who served as members of my qualifying exams committee and deemed me fit for PhD candidacy. My collaborators, Drs. Guntram von Kiparski from the Environmental Sciences Department and Samuel Webb from the Stanford Synchrotron Radiation Lightsource, were instrumental in supplying me with the skills and knowledge necessary to perform the many analytical tests that made this an interdisciplinary endeavor. -
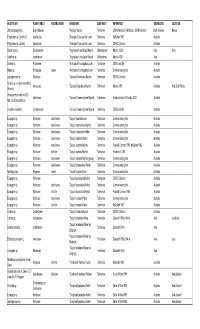
Sorted by Moth Species
HOST PLANT PLANT FAMILY FEEDING NICHE HERBIVORE SUBFAMILY REFERENCE GEOREGION LOCATION Jatropha gossypifolia Euphorbiaceae "Amorbia" depicta Tortricinae CSIRO Mexican Field Station; USNM collection North America Meixco Polystichum sp. ("prolifera") Aspidiaceae "Anisogona" placoxantha Lower Tortricinae McQuillan 1992 Australia Polystichum sp. (as fern) Aspidiaceae "Anisogona" placoxantha Lower Tortricinae CSIRO Collection Australia Cordia myxa L. Boraginaceae "Argyroploce" cenchropis Meyrick Olethreutinae Meyrick 1920a Asia India Acanthus sp. Acanthaceae "Argyroploce" vinculigera Meyrick Olethreutinae Meyrick 1939 Asia Banksia sp. Proteaceae "Arotrophora" cosmoplaca Lower Tortricinae CSIRO card file Australia Hakea sp. Proteaceae leaves "Arotrophora" cosmoplaca Lower Tortricinae Common rearing files Australia Leptospermum sp. Myrtaceae "Cacoecia" desmotana Meyrick Tortricinae CSIRO Collection Australia Senecio sp. (as plant resembling Asteraceae "Cacoecia" jugicolana Meyrick Tortricinae Meyrick 1881 Australia New South Wales Senecio) Osteospermum ecklonis (DC.) Asteraceae "Cacoecia" mnemosynana Meyrick Tortricinae Herbison-Evans & Crossley 2003 Australia Norl. (as Dimorphotheca) Goodenia ovata Sm. Goodeniaceae "Cacoecia" mnemosynana Meyrick Tortricinae CSIRO card file Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" ceramica Lower Tortricinae Common rearing files Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" cnaphalodes Meyrick Tortricinae Common rearing files Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" constrictana