Persistence and Restoration of the Patency of the Left Duct of Cuvier
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Anatomy of the Rectum and Anal Canal
BASIC SCIENCE identify the rectosigmoid junction with confidence at operation. The anatomy of the rectum The rectosigmoid junction usually lies approximately 6 cm below the level of the sacral promontory. Approached from the distal and anal canal end, however, as when performing a rigid or flexible sigmoid- oscopy, the rectosigmoid junction is seen to be 14e18 cm from Vishy Mahadevan the anal verge, and 18 cm is usually taken as the measurement for audit purposes. The rectum in the adult measures 10e14 cm in length. Abstract Diseases of the rectum and anal canal, both benign and malignant, Relationship of the peritoneum to the rectum account for a very large part of colorectal surgical practice in the UK. Unlike the transverse colon and sigmoid colon, the rectum lacks This article emphasizes the surgically-relevant aspects of the anatomy a mesentery (Figure 1). The posterior aspect of the rectum is thus of the rectum and anal canal. entirely free of a peritoneal covering. In this respect the rectum resembles the ascending and descending segments of the colon, Keywords Anal cushions; inferior hypogastric plexus; internal and and all of these segments may be therefore be spoken of as external anal sphincters; lymphatic drainage of rectum and anal canal; retroperitoneal. The precise relationship of the peritoneum to the mesorectum; perineum; rectal blood supply rectum is as follows: the upper third of the rectum is covered by peritoneum on its anterior and lateral surfaces; the middle third of the rectum is covered by peritoneum only on its anterior 1 The rectum is the direct continuation of the sigmoid colon and surface while the lower third of the rectum is below the level of commences in front of the body of the third sacral vertebra. -

Coronary Arterial Development Is Regulated by a Dll4-Jag1-Ephrinb2 Signaling Cascade
RESEARCH ARTICLE Coronary arterial development is regulated by a Dll4-Jag1-EphrinB2 signaling cascade Stanislao Igor Travisano1,2, Vera Lucia Oliveira1,2, Bele´ n Prados1,2, Joaquim Grego-Bessa1,2, Rebeca Pin˜ eiro-Sabarı´s1,2, Vanesa Bou1,2, Manuel J Go´ mez3, Fa´ tima Sa´ nchez-Cabo3, Donal MacGrogan1,2*, Jose´ Luis de la Pompa1,2* 1Intercellular Signalling in Cardiovascular Development and Disease Laboratory, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), Madrid, Spain; 2CIBER de Enfermedades Cardiovasculares, Madrid, Spain; 3Bioinformatics Unit, Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain Abstract Coronaries are essential for myocardial growth and heart function. Notch is crucial for mouse embryonic angiogenesis, but its role in coronary development remains uncertain. We show Jag1, Dll4 and activated Notch1 receptor expression in sinus venosus (SV) endocardium. Endocardial Jag1 removal blocks SV capillary sprouting, while Dll4 inactivation stimulates excessive capillary growth, suggesting that ligand antagonism regulates coronary primary plexus formation. Later endothelial ligand removal, or forced expression of Dll4 or the glycosyltransferase Mfng, blocks coronary plexus remodeling, arterial differentiation, and perivascular cell maturation. Endocardial deletion of Efnb2 phenocopies the coronary arterial defects of Notch mutants. Angiogenic rescue experiments in ventricular explants, or in primary human endothelial cells, indicate that EphrinB2 is a critical effector of antagonistic Dll4 and Jag1 functions in arterial morphogenesis. Thus, coronary arterial precursors are specified in the SV prior to primary coronary plexus formation and subsequent arterial differentiation depends on a Dll4-Jag1-EphrinB2 signaling *For correspondence: [email protected] (DMG); cascade. [email protected] (JLP) Competing interests: The authors declare that no Introduction competing interests exist. -

Abnormal Embryonic Lymphatic Vessel Development in Tie1 Hypomorphic Mice Xianghu Qu, Kevin Tompkins, Lorene E
© 2014. Published by The Company of Biologists Ltd | Development (2014) 141, 1417 doi:10.1242/dev.108969 CORRECTION Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice Xianghu Qu, Kevin Tompkins, Lorene E. Batts, Mira Puri and H. Scott Baldwin There was an error published in Development 137, 1285-1295. Author name H. Scott Baldwin was incomplete. The correct author list appears above. The authors apologise to readers for this mistake. 1417 RESEARCH ARTICLE 1285 Development 137, 1285-1295 (2010) doi:10.1242/dev.043380 © 2010. Published by The Company of Biologists Ltd Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice Xianghu Qu1, Kevin Tompkins1, Lorene E. Batts1, Mira Puri2 and Scott Baldwin1,3,* SUMMARY Tie1 is an endothelial receptor tyrosine kinase that is essential for development and maintenance of the vascular system; however, the role of Tie1 in development of the lymphatic vasculature is unknown. To address this question, we first documented that Tie1 is expressed at the earliest stages of lymphangiogenesis in Prox1-positive venous lymphatic endothelial cell (LEC) progenitors. LEC Tie1 expression is maintained throughout embryonic development and persists in postnatal mice. We then generated two lines of Tie1 mutant mice: a hypomorphic allele, which has reduced expression of Tie1, and a conditional allele. Reduction of Tie1 levels resulted in abnormal lymphatic patterning and in dilated and disorganized lymphatic vessels in all tissues examined and in impaired lymphatic drainage in embryonic skin. Homozygous hypomorphic mice also exhibited abnormally dilated jugular lymphatic vessels due to increased production of Prox1-positive LECs during initial lymphangiogenesis, indicating that Tie1 is required for the early stages of normal lymphangiogenesis. -

CCM2 and CCM3 Proteins Contribute to Vasculogenesis and Angiogenesis in Human Placenta
Histol Histopathol (2009) 24: 1287-1294 Histology and http://www.hh.um.es Histopathology Cellular and Molecular Biology CCM2 and CCM3 proteins contribute to vasculogenesis and angiogenesis in human placenta Gamze Tanriover1, Yasemin Seval1, Leyla Sati1, Murat Gunel2 and Necdet Demir1 1Department of Histology and Embryology, Akdeniz University, School of Medicine, Antalya, Turkey and 2 Department of Neurosurgery, Yale University, School of Medicine, New Haven, CT, USA Summary. Placenta as an ideal model to study Introduction angiogenic mechanisms have been established in previous studies. There are two processes, The placenta is a multifaceted organ that plays a vasculogenesis and angiogenesis, involved in blood critical role in maintaining and protecting the developing vessel formation during placental development. fetus. Normal development and function of the placenta Therefore, blood vessel formation is a crucial issue that requires extensive vasculogenesis and subsequent might cause vascular malformations. One of the vascular angiogenesis, in both maternal and fetal tissues. malformations is cerebral cavernous malformation Vasculogenesis is the formation of the primitive vascular (CCM) in the central nervous system, consisting of network de novo from progenitor cells, and angiogenesis endothelium-lined vascular channels without intervening is identified as the extension of blood vessels from normal brain parenchyma. Three CCM loci have been preexisting vascular structures (Demir et al., 1989, 2006; mapped as Ccm1, Ccm2, Ccm3 genes in CCM. In order Geva et al., 2002; Charnock-Jones et al., 2004). Many to investigate whether CCM proteins participate in blood factors, such as vascular endothelial growth factor vessel formation, we report here the expression patterns (VEGF), angiopoietins (Angpt-1 and -2) and their of CCM2 and CCM3 in developing and term human receptors are involved in the molecular regulation of placenta by means of immunohistochemistry and these diverse developmental steps. -

Anatomical Planes in Rectal Cancer Surgery
DOI: 10.4274/tjcd.galenos.2019.2019-10-2 Turk J Colorectal Dis 2019;29:165-170 REVIEW Anatomical Planes in Rectal Cancer Surgery Rektum Kanser Cerrahisinde Anatomik Planlar Halil İbrahim Açar, Mehmet Ayhan Kuzu Ankara University Faculty of Medicine, Department of General Surgery, Ankara, Turkey ABSTRACT This review outlines important anatomical landmarks not only for rectal cancer surgery but also for pelvic exentration. Keywords: Anorectal anatomy, pelvic anatomy, surgical anatomy of rectum ÖZ Pelvis anatomisini derleme halinde özetleyen bu makale rektum kanser cerrahisi ve pelvik ezantrasyon için önemli topografik noktaları gözden geçirmektedir. Anahtar Kelimeler: Anorektal anatomi, pelvik anatomi, rektumun cerrahi anatomisi Introduction Surgical Anatomy of the Rectum The rectum extends from the promontory to the anal canal Pelvic Anatomy and is approximately 12-15 cm long. It fills the sacral It is essential to know the pelvic anatomy because of the concavity and ends with an anal canal 2-3 cm anteroinferior intestinal and urogenital complications that may develop to the tip of the coccyx. The rectum contains three folds in after the surgical procedures applied to the pelvic region. the coronal plane laterally. The upper and lower are convex The pelvis, encircled by bone tissue, is surrounded by the to the right, and the middle is convex to the left. The middle main vessels, ureters, and autonomic nerves. Success in the fold is aligned with the peritoneal reflection. Intraluminal surgical treatment of pelvic organs is only possible with a projections of the lower boundaries of these folds are known as Houston’s valves. Unlike the sigmoid colon, taenia, good knowledge of the embryological development of the epiploic appendices, and haustra are absent in the rectum. -

The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration
REVIEWS The evolving cardiac lymphatic vasculature in development, repair and regeneration Konstantinos Klaourakis 1,2, Joaquim M. Vieira 1,2,3 ✉ and Paul R. Riley 1,2,3 ✉ Abstract | The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease. The circulatory system of vertebrates is composed of two after MI. In this Review, we summarize the current complementary vasculatures, the blood and lymphatic knowledge on the development, structure and function vascular systems1. The blood vasculature is a closed sys- of the cardiac lymphatic vasculature, with an emphasis tem responsible for transporting gases, fluids, nutrients, on breakthroughs over the past 5 years in the study of metabolites and cells to the tissues2. This extravasation of cardiac lymphatic heterogeneity in mice and zebrafish. -

CASE REPORT Spinal Cord Infarction Following Endoscopic Variceal Ligation
Spinal Cord (2008) 46, 241–242 & 2008 International Spinal Cord Society All rights reserved 1362-4393/08 $30.00 www.nature.com/sc CASE REPORT Spinal cord infarction following endoscopic variceal ligation K Tofuku, H Koga, T Yamamoto, K Yone and S Komiya Department of Orthopaedic Surgery, Kagoshima Graduate School of Medical and Dental Sciences, Kagoshima, Japan Study design: A case report of spinal cord infarction following endoscopic variceal ligation. Objectives: To describe an exceedingly rare case of spinal cord infarction following endoscopic variceal ligation. Setting: Department of Orthopaedic Surgery, Kagoshima, Japan. Methods: A 75-year-old woman with cirrhosis caused by hepatitis C virus, who was admitted to our hospital for the treatment of esophageal varices, experienced numbness of the hands and lower extremities bilaterally following an endoscopic variceal ligation procedure. Sensory and motor dysfunction below C6 level progressed rapidly, resulting in inability to move the lower extremities the following day. Magnetic resonance imaging of the spine revealed abnormal spinal cord signal on T2-weighted images from approximately C6 through T5 levels, which was diagnosed as spinal cord infarction. Results: The patient underwent hyperbaric oxygen treatment. Her symptoms and signs related to spinal cord infarction gradually remitted, and nearly complete disappearance of neurological deficits was noted within 3 months after the start of treatment. Conclusion: We speculate that the pathogenesis of the present case may have involved congestion of the abdominal–epidural–spinal cord venous network owing to ligation of esophageal varices and increased thoracoabdominal cavity pressure. Spinal Cord (2008) 46, 241–242; doi:10.1038/sj.sc.3102092; published online 19 June 2007 Keywords: endoscopic variceal ligation; hyperbaric oxygen; spinal cord infarction Introduction The spectrum of etiologies of spinal cord infarction is as On physical examination, her right upper extremity diverse as that for cerebral infarction. -

Development of HEART 4-VEINS
Development of brachiocephalic veins 1. Right brachiocephalic vein is formed by cranial part of right anterior cardinal vein and 2. Left brachiocephalic is formed by cranial part of left anterior cardinal vein and the interant.cardinal anastomosis. Development of superior vena cava 1. The part up to the opening of vena azygos develops from caudal part of right ant.cardinal vein and 2. The part below the opening (intrapericardial part) is formed by the right common cardinal vein. Development of azygos and hemiazygos veins A. 1. Vena azygos develops from right azygos line vein and 2. The arch of vena azygos is formed by the cranial end of right postcardinal vein. B. Hemiazygos veins are formed by the left azygos line vein. Development of Inferior vena cava Inferior vena cava is formed, from below upwards by: 1. Begins by the union of the two common iliac veins (postcardinal veins), 2. Right supracardinal, 3. Right supra-subcardinal anastomosis, 4. Right subcardinal, 5. New formation (hepatic segment) and 6. Hepatocardiac channel (terminal part of right vitelline vein). Congenital anomalies • Double inferior vena cava • Absence • Left SVC • Double SVC DEVELOPMENT OF PORTAL VEIN 1. The portal vein is formed behind the neck of pancreas by the union of superior mesentric and splenic vein to the left vitelline vein. 2. The part of the portal vein which is behind the Ist part of duodenum is formed by middle dorsal transverse anastomosis. 3. Part of portal vein which is in the free margin of lesser omentum is formed by cranial or distal part of right vitelline vein. -

Anatomy of the Large Blood Vessels-Veins
Anatomy of the large blood vessels-Veins Cardiovascular Block - Lecture 4 Color index: !"#$%&'(& !( "')*+, ,)-.*, $()/ Don’t forget to check the Editing File !( 0*"')*+, ,)-.*, $()/ 1$ ($&*, 23&%' -(0$%"'&-$(4 *3#)'('&-$( Objectives: ● Define veins, and understand the general principles of venous system. ● Describe the superior & inferior Vena Cava and their tributaries. ● List major veins and their tributaries in the body. ● Describe the Portal Vein. ● Describe the Portocaval Anastomosis Veins ◇ Veins are blood vessels that bring blood back to the heart. ◇ All veins carry deoxygenated blood. with the exception of the pulmonary veins(to the left atrium) and umbilical vein(umbilical vein during fetal development). Vein can be classified in two ways based on Location Circulation ◇ Superficial veins: close to the surface of the body ◇ Veins of the systemic circulation: NO corresponding arteries Superior and Inferior vena cava with their tributaries ◇ Deep veins: found deeper in the body ◇ Veins of the portal circulation: With corresponding arteries Portal vein Superior Vena Cava ◇Formed by the union of the right and left Brachiocephalic veins. ◇Brachiocephalic veins are formed by the union of internal jugular and subclavian veins. Drains venous blood from : ◇ Head & neck ◇ Thoracic wall ◇ Upper limbs It Passes downward and enter the right atrium. Receives azygos vein on its posterior aspect just before it enters the heart. Veins of Head & Neck Superficial veins Deep vein External jugular vein Anterior Jugular Vein Internal Jugular Vein Begins just behind the angle of mandible It begins in the upper part of the neck by - It descends in the neck along with the by union of posterior auricular vein the union of the submental veins. -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -

The Placenta
The placenta Learning module Developed by Carolyn Hammer Edited by Fabien Giroux Diagrams By Dr Julien Yockell Lelievre where indicated The placenta – Learning module Table of content 1) Introduction……………………………………………………………………….3 2) Anatomy and Physiology………………………………………………………..6 3) Roles and Functions……………………………………………………………17 4) Development and formation…………………………………..…………….…27 5) What happens after birth……………………………………………….……...34 6) What happens when things go wrong.……………………………………….36 7) Interesting facts about pregnancy………………….…………………………46 2 The placenta – Learning module Introduction 3 The placenta – Learning module What is the placenta? •The placenta is a “vascular (supplied with blood vessels) organ in most mammals that unites the fetus to the uterus of the mother. It mediates the metabolic exchanges of the developing individual through an intimate association of embryonic tissues and of certain uterine tissues, serving the functions of nutrition, respiration, and excretion.” (Online Britannica encyclopaedia) •As the fetus is in full development, it requires a certain amount of gases and nutrients to help support its growth. Because the fetus is unable to do so on its own, the placenta provides these gases and nutrients throughout pregnancy. http://health.allrefer.com/health/plac enta-abruptio-placenta.html 4 The placenta – Learning module What are the main roles of the placenta? •The placenta provides the connection between fetus and mother in order to help carry out many different functions that the growing baby is incapable to do so alone. During pregnancy, the placenta has 6 main roles to maintain good health and a good environment for the growing child: •Respiration •Nutrition •Excretion •Protection •Endocrine •Immunity 5 The placenta – Learning module Anatomy and physiology 6 The placenta – Learning module Structure •A placenta is an organ of round or oval shape that is relatively flat. -
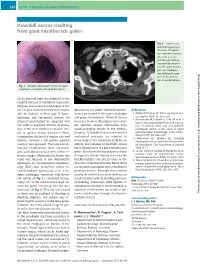
Downhill Varices Resulting from Giant Intrathoracic Goiter
E40 UCTN – Unusual cases and technical notes Downhill varices resulting from giant intrathoracic goiter Fig. 2 Sagittal com- puted tomography of the chest. The goiter was immense, reaching the aortic arch, sur- rounding the trachea and partially compres- sing the upper esopha- gus. The esophagus was additionally com- pressed by anterior spinal spondylophytes. Fig. 1 Multiple submucosal veins in the upper esophagus, consistent with downhill varices. An 82-year-old man was admitted to the hospital because of substernal chest pain, dyspnea, and occasional dysphagia to sol- ids. His past medical history was remark- geal varices are called “downhill varices”, References able for diabetes mellitus type II, hyper- as they are located in the upper esophagus 1 Kotfila R, Trudeau W. Extraesophageal vari- – lipidemia, and Parkinson’s disease. On and project downwards. Downhill varices ces. Dig Dis 1998; 16: 232 241 2 Basaranoglu M, Ozdemir S, Celik AF et al. A occur as a result of shunting in cases of up- physical examination he appeared frail case of fibrosing mediastinitis with obstruc- but with no apparent distress. Examina- per systemic venous obstruction from tion of superior vena cava and downhill tion of the neck showed no masses, stri- space-occupying lesions in the medias- esophageal varices: a rare cause of upper dor or jugular venous distension. Heart tinum [2,3]. Downhill varices as a result of gastrointestinal hemorrhage. J Clin Gastro- – examination disclosed a regular rate and mediastinal processes are reported to enterol 1999; 28: 268 270 3 Calderwood AH, Mishkin DS. Downhill rhythm; however a 2/6 systolic ejection occur in up to 50% of patients [3,4].