Form 20-F 2020 | 1 Part I Item 3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Hexavalent Dtap/IPV/Hib/Hepb Combination Vaccine: Information for Healthcare Practitioners About the Neonatal Selective
The hexavalent DTaP/IPV/Hib/HepB combination vaccine Information for healthcare practitioners about the neonatal selective immunisation programme for babies at risk of hepatitis B The Hexavalent DTaP/IPV/Hib/HepB combination vaccine: Information for Healthcare Practitioners (selective programme) About Public Health England Public Health England exists to protect and improve the nation’s health and wellbeing, and reduce health inequalities. We do this through world-leading science, research, knowledge and intelligence, advocacy, partnerships and the delivery of specialist public health services. We are an executive agency of the Department of Health and Social Care, and a distinct delivery organisation with operational autonomy. We provide government, local government, the NHS, Parliament, industry and the public with evidence-based professional, scientific and delivery expertise and support. Public Health England Wellington House 133-155 Waterloo Road London SE1 8UG Tel: 020 7654 8000 www.gov.uk/phe Twitter: @PHE_uk Facebook: www.facebook.com/PublicHealthEngland For queries relating to this document, please contact: [email protected] © Crown copyright 2020 You may re-use this information (excluding logos) free of charge in any format or medium, under the terms of the Open Government Licence v3.0. To view this licence, visit OGL. Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned. First published November 2017 This updated version published February -

Top Twenty Pay-For-Delay Drugs: How Drug Industry Payoffs Delay Generics, Inflate Prices and Hurt Consumers
TOP TWENTY PAY-FOR-DELAY DRUGS: HOW DRUG INDUSTRY PAYOFFS DELAY GENERICS, INFLATE PRICES AND HURT CONSUMERS oo often, consumers are forced to shoulder a heavy financial burden, or even go without needed medicine, due to the high cost of brand-name drugs. Our research indicates that one significant cause is Tthe practice called “pay for delay,” which inflates the drug prices paid by tens of millions of Americans. In a pay-for-delay deal, a brand-name drug company pays off a would-be competitor to delay it from selling a generic version of the drug. Without any competition, the brand-name company can continue demanding high prices for its drug. This list of 20 drugs known to be impacted by pay-for-delay deals represents the tip of the iceberg. Annual reports by the Federal Trade Commission (FTC) indicate that generic versions of as many as 142 brand-name drugs have been delayed by pay-for-delay arrangements between drug manufacturers since 2005.1 However, because the details of these deals rarely become public, consumers have largely been kept in the dark about the extent of the problem. Information about these twenty specific drugs affected by pay-for-delay deals has been made public thanks to legal challenges brought by the FTC, consumer class action lawsuits, research by legal experts, and public disclosures by drug makers. Key findings of our analysis of these 20 drugs impacted by pay-for-delay deals: . This practice has held back generic . These brand-name drugs cost medicines used by patients with a wide 10 times more than their generic range of serious or chronic conditions, equivalents, on average, and as much as ranging from cancer and heart disease, to 33 times more. -

Current and Future Natural Gas Demand in China and India
Global Gas/LNG Research Current and Future Natural Gas Demand in China and India By Miranda Wainberg Senior Energy Advisor Michelle Michot Foss, Ph.D. Chief Energy Economist and Program Manager Gürcan Gülen, Ph.D. Senior Energy Economist and Research Scientist Daniel Quijano Economist and Research Associate April 2017 April 2017, BEG/CEE China/India Gas Demand, Page 1 TABLE OF CONTENTS ESSENTIAL ACRONYMNS, UNITS AND CONVERSIONS ................................................................................................... 5 ACKNOWLEDGMENTS .................................................................................................................................................... 6 PREFACE ......................................................................................................................................................................... 7 INTRODUCTION ............................................................................................................................................................. 8 MACROECONOMIC CONTEXT FOR NATURAL GAS IN CHINA AND INDIA ...................................................................... 9 Composition of GDP and Employment Structure ...................................................................................................... 9 GDP Growth and Industrial Structures in China and India ...................................................................................... 11 Industrial overcapacity and debt in China ......................................................................................................... -
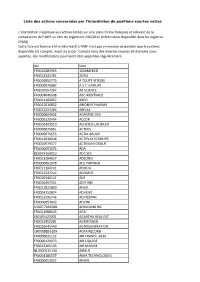
Liste Des Actions Concernées Par L'interdiction De Positions Courtes Nettes
Liste des actions concernées par l'interdiction de positions courtes nettes L’interdiction s’applique aux actions listées sur une plate-forme française et relevant de la compétence de l’AMF au titre du règlement 236/2012 (information disponible dans les registres ESMA). Cette liste est fournie à titre informatif. L'AMF n'est pas en mesure de garantir que le contenu disponible est complet, exact ou à jour. Compte tenu des diverses sources de données sous- jacentes, des modifications pourraient être apportées régulièrement. Isin Nom FR0010285965 1000MERCIS FR0013341781 2CRSI FR0010050773 A TOUTE VITESSE FR0000076887 A.S.T. GROUPE FR0010557264 AB SCIENCE FR0004040608 ABC ARBITRAGE FR0013185857 ABEO FR0012616852 ABIONYX PHARMA FR0012333284 ABIVAX FR0000064602 ACANTHE DEV. FR0000120404 ACCOR FR0010493510 ACHETER-LOUER.FR FR0000076861 ACTEOS FR0000076655 ACTIA GROUP FR0011038348 ACTIPLAY (GROUPE) FR0010979377 ACTIVIUM GROUP FR0000053076 ADA BE0974269012 ADC SIIC FR0013284627 ADEUNIS FR0000062978 ADL PARTNER FR0011184241 ADOCIA FR0013247244 ADOMOS FR0010340141 ADP FR0010457531 ADTHINK FR0012821890 ADUX FR0004152874 ADVENIS FR0013296746 ADVICENNE FR0000053043 ADVINI US00774B2088 AERKOMM INC FR0011908045 AG3I ES0105422002 AGARTHA REAL EST FR0013452281 AGRIPOWER FR0010641449 AGROGENERATION CH0008853209 AGTA RECORD FR0000031122 AIR FRANCE -KLM FR0000120073 AIR LIQUIDE FR0013285103 AIR MARINE NL0000235190 AIRBUS FR0004180537 AKKA TECHNOLOGIES FR0000053027 AKWEL FR0000060402 ALBIOMA FR0013258662 ALD FR0000054652 ALES GROUPE FR0000053324 ALPES (COMPAGNIE) -

Occupational Characteristics and Management Measures of Sporadic
Occupational characteristics and management measures of sporadic COVID-19 outbreaks from June 2020 to January 2021 in China: the importance of tracking down “patient zero” Maohui Feng 1,a, Qiong Ling 2,a, Jun Xiong 3, Anne Manyande 4, Weiguo Xu 5, Boqi Xiang 6,* 1 Department of Gastrointestinal Surgery, Wuhan Peritoneal Cancer Clinical Medical Research Center, Zhongnan Hospital of Wuhan University, Hubei Key Laboratory of Tumor Biological Behaviors and Hubei Cancer Clinical Study Center, Wuhan 430071, Hubei, PR China; 2 Department of Anesthesiology, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510120, Guangdong, PR China; 3 Hepatobiliary Surgery Center, Union Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, PR China; 4 School of Human and Social Sciences, University of West London, London, United Kingdom; 5 Department of Orthopedics, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, PR China; 6 School of Public Health, University of Rutgers, New Brunswick, NJ 08854, USA. a These authors have contributed equally to this work. *Correspondence: Boqi Xiang, E-mail: [email protected] Abstract There are occupational disparities in the risk of contracting COVID-19. Occupational characteristics and work addresses play key roles in tracking down “patient zero”. The present descriptive analysis for occupational characteristics and management measures of sporadic COVID-19 outbreaks from June to December 2020 in China offers important new information to the international community at this stage of the pandemic. These data suggest that Chinese measures including tracking down “patient zero”, launching mass COVID-19 testing in the SARS-CoV-2-positive areas, designating a new high or medium-risk area, locking down the corresponding community or neighborhood in response to new COVID-19 cases and basing individual methods of protection on science, are effective in reducing transmission of the highly contagious SARS-CoV-2 across China. -

Download File (Pdf)
2021 FORUM REPORT COVID-19 in Africa one year on: Impact and Prospects MO IBRAHIM FOUNDATION 2021 FORUM REPORT COVID-19 in Africa one year on: Impact and Prospects MO IBRAHIM FOUNDATION Foreword by Mo Ibrahim Notwithstanding these measures, on current projections Founder and Chair of the Mo Ibrahim Africa might not be adequately covered before 2023. Foundation (MIF) Vaccinating Africa is an urgent matter of global security and all the generous commitments made by Africa’s partners must now be delivered. Looking ahead - and inevitably there will be future pandemics - Africa needs to significantly enhance its Over a year ago, the emergence and the spread of COVID-19 homegrown vaccine manufacturing capacity. shook the world and changed life as we knew it. Planes were Africa’s progress towards its development agendas was off grounded, borders were closed, cities were shut down and course even before COVID-19 hit and recent events have people were told to stay at home. Other regions were hit created new setbacks for human development. With very earlier and harder, but Africa has not been spared from the limited access to remote learning, Africa’s youth missed out pandemic and its impact. on seven months of schooling. Women and girls especially The 2021 Ibrahim Forum Report provides a comprehensive are facing increased vulnerabilities, including rising gender- analysis of this impact from the perspectives of health, based violence. society, politics, and economics. Informed by the latest data, The strong economic and social impacts of the pandemic it sets out the challenges exposed by the pandemic and the are likely to create new triggers for instability and insecurity. -

Controlling Pregnancy: Fred Lyman Adair and the Influence of Eugenics on the Development of Prenatal Care
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine January 2019 Controlling Pregnancy: Fred Lyman Adair And The nflueI nce Of Eugenics On The evelopmeD nt Of Prenatal Care Florence Hsiao Follow this and additional works at: https://elischolar.library.yale.edu/ymtdl Recommended Citation Hsiao, Florence, "Controlling Pregnancy: Fred Lyman Adair And The nflueI nce Of Eugenics On The eD velopment Of Prenatal Care" (2019). Yale Medicine Thesis Digital Library. 3504. https://elischolar.library.yale.edu/ymtdl/3504 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. Controlling Pregnancy: Fred Lyman Adair and the Influence of Eugenics on the Development of Prenatal Care A Thesis Submitted to the Yale University School of Medicine In Partial Fulfillment of the Requirements for the Degree of Doctor of Medicine By Florence Hsiao Class of 2019 Abstract This thesis examines the development of prenatal care in the United States in the early 1900s by focusing on the life and career of Fred Lyman Adair who, as an obstetrician and eugenicist, played a significant role in shaping prenatal care into what it is today. Although prenatal care was a product of infant welfare activists and public health officials, obstetricians like Adair who struggled to establish obstetrics as a legitimate specialty, saw an opportunity in prenatal care to pathologize pregnancy and elevate their specialty. -

Air Liquide, Airbus E Il Gruppo ADP Collaborano Per Preparare Gli
COMUNICATO STAMPA CONGIUNTO Parigi, 21 Giugno 2021 Air Liquide, Airbus e Groupe ADP insieme per preparare gli aeroporti di Parigi all’era dell’idrogeno Air Liquide, Airbus e Groupe ADP hanno firmato un protocollo d’intesa per preparare l’arrivo dell'idrogeno negli aeroporti entro il 2035 nell'ambito dello sviluppo di velivoli commerciali alimentati a idrogeno. I partner intendono fare leva sulle rispettive competenze per supportare la decarbonizzazione del trasporto aereo e definire le esigenze concrete e le opportunità che l'idrogeno può apportare al settore aeronautico. Questa partnership riflette l'ambizione comune dei tre gruppi di contribuire allo sviluppo di una filiera francese innovativa e strategica in vista di un'aviazione globale senza emissioni di carbonio. Per prepararsi all'arrivo dei primi aerei di linea a idrogeno entro il 2035, gli aeroporti dovranno essere adattati, in particolare per includere la specificità della fornitura di idrogeno liquido. La partnership annunciata oggi si concentra sulla realizzazione di studi di fattibilità finalizzati allo sviluppo di queste infrastrutture. Come primo step, verrà avviato uno studio che coinvolgerà un panel rappresentativo di circa 30 aeroporti in tutto il mondo per valutare le potenziali configurazioni per la produzione, fornitura e distribuzione di idrogeno liquido. Verranno poi elaborati scenari dettagliati e i piani per i due principali aeroporti parigini: Paris-Charles de Gaulle e Paris-Orly. Questi scenari saranno essenziali nella definizione dell'infrastruttura richiesta, inclusi il dimensionamento e la posizione, e per identificare e integrare i vincoli relativi sia agli standard di sicurezza industriale che alle norme previste per l’aeronautica. La partnership riunisce competenze complementari con l'ambizione di supportare, a partire da oggi, la trasformazione degli aeroporti e aprire la strada a una nuova era di viaggi aerei sostenibili. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

WO 2017/173059 Al 5 October 2017 (05.10.2017) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2017/173059 Al 5 October 2017 (05.10.2017) P O P C T (51) International Patent Classification: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, C12N 9/26 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KH, KN, (21) International Application Number: KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, PCT/US20 17/024981 MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, (22) International Filing Date: NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, 30 March 2017 (30.03.2017) RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, (25) Filing Language: English ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 62/3 15,400 30 March 2016 (30.03.2016) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/457,584 10 February 2017 (10.02.2017) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, 15/473,994 30 March 2017 (30.03.2017) US TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (71) Applicant: AMICUS THERAPEUTICS, INC. -

An Overview: Innovation in Plant Breeding
An Overview: Innovation in Plant Breeding Modern plant breeding blends traditional ways of developing crops with the latest in science and technology to achieve improved crops – enabling more choices for both farmers and consumers, and producing crops that can better cope with evolving pests, diseases and a changing climate. The Basics What: The simplest definition of plant breeding is crossing two plants Most of the fruits, vegetables, and to produce offspring that share the best characteristics of the two grains that we eat today are the parent plants. Breeders make crosses then select which offspring to advance in the pipeline based on their desired characteristics. result of generations of plant breeding. About 5,000 years ago, Why: Even the earliest farmers understood that, in order to survive, watermelons were only they needed plant varieties specifically adapted to their environmental conditions and cultivated to produce the best foods to nourish their 2 inches in diameter livestock and communities. and had a bitter taste, vastly How: Through generations of research and discovery, plant breeding has advanced beyond selecting a parent plant simply based on its different from appearance. It now includes an in-depth understanding of plants’ the large, genetic makeup, enabling breeders to better predict which plants will sweet-tasting have the highest probability of success in the field and the grocery fruit we enjoy store before making a cross. today. The Background The earliest farmers were plant breeders who understood the value of identifying crops that showed beneficial characteristics to plant in future seasons. Later, they learned they could cross two plants to develop an even better plant. -

Factset-Top Ten-0521.Xlsm
Pax International Sustainable Economy Fund USD 7/31/2021 Port. Ending Market Value Portfolio Weight ASML Holding NV 34,391,879.94 4.3 Roche Holding Ltd 28,162,840.25 3.5 Novo Nordisk A/S Class B 17,719,993.74 2.2 SAP SE 17,154,858.23 2.1 AstraZeneca PLC 15,759,939.73 2.0 Unilever PLC 13,234,315.16 1.7 Commonwealth Bank of Australia 13,046,820.57 1.6 L'Oreal SA 10,415,009.32 1.3 Schneider Electric SE 10,269,506.68 1.3 GlaxoSmithKline plc 9,942,271.59 1.2 Allianz SE 9,890,811.85 1.2 Hong Kong Exchanges & Clearing Ltd. 9,477,680.83 1.2 Lonza Group AG 9,369,993.95 1.2 RELX PLC 9,269,729.12 1.2 BNP Paribas SA Class A 8,824,299.39 1.1 Takeda Pharmaceutical Co. Ltd. 8,557,780.88 1.1 Air Liquide SA 8,445,618.28 1.1 KDDI Corporation 7,560,223.63 0.9 Recruit Holdings Co., Ltd. 7,424,282.72 0.9 HOYA CORPORATION 7,295,471.27 0.9 ABB Ltd. 7,293,350.84 0.9 BASF SE 7,257,816.71 0.9 Tokyo Electron Ltd. 7,049,583.59 0.9 Munich Reinsurance Company 7,019,776.96 0.9 ASSA ABLOY AB Class B 6,982,707.69 0.9 Vestas Wind Systems A/S 6,965,518.08 0.9 Merck KGaA 6,868,081.50 0.9 Iberdrola SA 6,581,084.07 0.8 Compagnie Generale des Etablissements Michelin SCA 6,555,056.14 0.8 Straumann Holding AG 6,480,282.66 0.8 Atlas Copco AB Class B 6,194,910.19 0.8 Deutsche Boerse AG 6,186,305.10 0.8 UPM-Kymmene Oyj 5,956,283.07 0.7 Deutsche Post AG 5,851,177.11 0.7 Enel SpA 5,808,234.13 0.7 AXA SA 5,790,969.55 0.7 Nintendo Co., Ltd.