GRDC Grains Research Update Proceedings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lucinda Watt Thesis
Sheep production on second generation, hardseeded annual pasture legumes fed as grazed and conserved forages in the Mediterranean- temperate environment of southern NSW Lucinda J Watt Bachelor of Animal Science (Hons. 1) (CSU, AUS) A thesis submitted to Charles Sturt University, Wagga Wagga, for the degree of Doctor of Philosophy (PhD) August 2019 CERTIFICATE OF AUTHORSHIP I hereby declare that this submission is my own work and to the best of my knowledge and belief, understand that it contains no material previously published or written by another person, nor material which to a substantial extent has been accepted for the award of any other degree or diploma at Charles Sturt University or any other educational institution, except where due acknowledgement is made in the thesis. Any contribution made to the research by colleagues with whom I have worked at Charles Sturt University or elsewhere during my candidature is fully acknowledged. I agree that this thesis be accessible for the purpose of study and research in accordance with normal conditions established by the Executive Director, Library Services, Charles Sturt University or nominee, for the care, loan and reproduction of thesis, subject to confidentiality provisions as approved by the University. ………………………….. Date: 08/08/2019 Lucinda J Watt I ACKNOWLEDGEMENTS This thesis would not have been possible without the help of some wonderful people who assisted me (whether it was big or small) in carrying out my experiments, reading and making edits on my thesis chapters, helping me further develop my knowledge and skill set, whilst providing some much needed emotional support throughout this experience. -

Bland Shire Council Bland Shire Council PO Box 21 PO Box 21 WEST WYALONG NSW 2671 WEST WYALONG NSW 2671
Ray Smith Jeff Stien General Manager Senior Economic Development & Tourism Advisor Bland Shire Council Bland Shire Council PO Box 21 PO Box 21 WEST WYALONG NSW 2671 WEST WYALONG NSW 2671 7 March 2018 The Hon Andrew Constance MP The Hon Melinda Pavey MP Minister for Transport and Infrastructure Minister for Roads, Maritime and Freight GPO Box 5341 GPO Box 5341 SYDNEY NSW 2001 SYDNEY NSW 2001 Dear Minister Constance and Minister Pavey Thank you for the opportunity for Bland Shire Council to provide a submission to the Future Transport 2056 NSW Draft Freight and Ports Plan. Bland Shire Council extends an invitation for Minister Constance and Minister Pavey and Transport NSW to visit the Bland Shire to see firsthand the transport task and the agricultural and mining activities that are in operation or that are being proposed in the Bland Shire. Bland Shire Council commends the NSW Government and Transport NSW for developing the following draft plans: 1. Draft Tourism and Transport Plan, Supporting the Visitor Economy October 2017 2. Regional NSW, Services and Infrastructure Plan 3. Draft Future Transport Strategy 2056 4. Draft Road Safety Plan 2021 5. NSW Draft Freight and Ports Plan Bland Shire Council has submitted comments on these plans and Bland Shire Council would like these comments to be taken into consideration with Bland Shire Councils submission to the NSW Draft Freight and Ports Plan. The Future Transport Plans mentions the use and adoption of new technologies and smart phones for example: • Technology is changing how we travel – and how we deliver transport. • Raising customer standards through technology. -

Goldenfields Water County
INDUSTRY CASE STUDIES GOLDENFIELDS WATER COUNTY THE PROJECT PROJECT SUMMARY After more than 70 years in operation, The Goldenfields Water County Council called for the existing pipelines connecting Reefton to Barmedman and Yiddah to Wyalong to be replaced. The two sections of pipeline were originally PROJECT R2B and Y2W Pipeline Replacement Project installed during the 1930’s to transfer water from the Murrumbidgee River to Temora and further on to Wyalong. CLIENT Goldenfields Water County Council Since that time the pipelines consisted of unlined mild steel and cement lined cast iron and had been subject to corrosion leading to a large number of leaks LOCATION Wyalong, New South Wales and breakages, reducing the efficiency and sustainability of the pipeline. THE SOLUTION DATE Deliveries completed August 2009 Viadux was contracted to supply 20 kilometres of TYTON Ductile Iron pipe and SUREFLOW fittings to replace the failing pipeline. 10km of TYTON DN375 DICL pipe SUPPLIED and 10km of TYTON DN300 DICL Pipe Viadux supplied ten kilometres of TYTON Ductile Iron pipe for the Reefton to Barmedman section and ten kilometres of TYTON Ductile Iron pipe for the Yiddah to Wyalong section. Included in this supply were SUREFLOW Ductile Iron fittings for the two sections including bends, tees, valves, couplings. THE RESULT The pipeline replacement program will secure water to towns including West Wyalong, Ungarie and Girral by delivering approximately 7ML of water per day. The new pipeline has been designed to allow for greater flows of water at significantly higher pressures than the previous pipeline to accommodate future growth in the region. -

Annual Report 2016-2017 Bland Shire Council Annual Report 2016/17
Annual Report 2016-2017 Bland Shire Council Annual Report 2016/17 CONTENTS INTRODUCTION 4 VISION, MISSION & VALUES 4 COMMON ACRONYMS USED IN BLAND SHIRE COUNCIL REPORTS AND DOCUMENTS 5 ABOUT THIS REPORT 7 SECTION 1: REPORT SUMMARY 8 REPORTING FRAMEWORK 8 MESSAGE FROM THE MAYOR & GENERAL MANAGER 10 THE YEAR IN REVIEW 12 SHIRE PROFILE 14 COUNCIL PROFILE 16 COUNCILLORS 21 REPRESENTATIVES ON STATE/REGIONAL ORGANISATIONS AND COUNCIL COMMITTEES 23 COUNCIL MEETINGS – DECISION MAKING 24 2016/2017 FINANCIAL SUMMARY 30 FINANCIAL SNAPSHOT 31 SECTION 2: STRATEGIC PRIORITIES 32 OUR PEOPLE: KEY ACHIEVEMENTS, PROGRAMS & PROJECTS 34 OUR PLACES: KEY ACHIEVEMENTS, PROGRAMS & PROJECTS 45 OUR INFRASTRUCTURE: KEY ACHIEVEMENTS, PROGRAMS & PROJECTS 51 OUR LEADERSHIP: KEY ACHIEVEMENTS, PROGRAMS & PROJECTS 57 OUR PROSPERITY: KEY ACHIEVEMENTS, PROGRAMS & PROJECTS 66 SECTION 3: STATUTORY INFORMATION 74 DETAILS OF OVERSEES VISITS BY COUNCILLORS, COUNCIL STAFF OR REPRESENTATIVES 74 DETAILS OF MAYORAL AND COUNCILLOR FEES, EXPENSES AND FACILITIES 74 CONTRACTS AWARDED 76 PRIVATE WORKS AND FINANCIAL ASSISTANCE 77 DETAILS OF EXTERNAL BODIES, COMPANIES AND PARTNERSHIPS 79 EEO MANAGEMENT PLAN 81 EXECUTIVE REMUNERATION PACKAGES 82 STORMWATER MANAGEMENT 83 Page 2 Bland Shire Council Annual Report 2016/17 REPORT ON CAPITAL WORKS PROJECTS 84 GOVERNMENT INFORMATION (PUBLIC ACCESS) ACT (GIPA) ANNUAL REPORT 88 PUBLIC INTEREST DISCLOSURES REPORT 95 ENVIRONMENTAL PLANNING AND ASSESSSMENT ACT 1979 96 CARERS RECOGNITION ACT 2010 96 SECTION 4: NON MANDATORY REPORTING AREAS 97 ASSET REPORTING 97 HUMAN RESOURCES ACTIVITIES 101 WHS MANAGEMENT SYSTEM 103 BUSINESS ACTIVITIES 106 DEVELOPMENT STATISTICS 107 NSW RURAL FIRE SERVICE (NSWRFS) 2016/2017 ANNUAL REPORT 108 ATTACHMENT 2: FINANCIAL STATEMENTS 111 Page 3 Bland Shire Council Annual Report 2016/17 INTRODUCTION Vision, Mission & Values Council’s strategic direction is supported by our vision, mission and values. -

1900 Miscellaneous Land Tenure in Western and Some of Central NSW Mentioned in the 1900 Government Gazette
Rusheen’s Website: www.rusheensweb.com 1900 Miscellaneous Land Tenure in Western and Some of Central NSW mentioned in the 1900 Government Gazette RUSHEEN CRAIG January 2012 Last updated: 4 November 2012 Copyright © 2012 Rusheen Craig Using the information from this document: Please note that the research on this web site is freely provided for personal use only. Site users have the author's permission to utilise this information in personal research, but any use of information and/or data in part or in full for republication in any printed or electronic format (regardless of commercial, non-commercial and/or academic purpose) must be attributed in full to Rusheen Craig. All rights reserved by Rusheen Craig. Miscellaneous Land Tenure Copyright © 2012, Rusheen Craig 1 of 240 Holder Lease Type and Qualification/Location/(Purpose) Area (Acres) Rental or No of Papers Type of Action Number Price (See Legend) £-s-d ABBOTT Louisa After Auction Sold at Corowa.LD & Psh-Mulwala. Co-Denison.Lot 5.Sec 39 …. …. 98-16210 Annulled Purchase a'BECKETT W.C. and a'BECKETT M.E. Preferential No 177; "Nelgowrie"; Central Division. 14,807 …. Occ1900-6564 PrOccL Granted Occupation License aBECKETT William Channing and POccL 177A Central Division. "Nelgowrie". 14,807 115-13-9 …. Pref Occupation aBECKETT Marsham Elwin License. A'BECKETT William Channing and OccL 177 Central Division. "Nelgowrie". 1,010 7-17-10 …. Renewal of Marsham Elwin Occupation License for 1901 ABERNETHY Harold CP 94-1 LD-Wellington.Psh-Guroba.Sec 42. Port 8. 40 …. 98-4768 Certificate of Conformity ABERNETHY Harold CP 95-9 LD-Wellington.Psh-Guroba.Sec 42. -

Integrated Regional Vulnerability Assessment: Riverina Murray
Integrated Regional Vulnerability Assessment: Riverina Murray Volume 2: Priority Sector Workshops – Summary Findings i © State of NSW, Office of Environment and Heritage. The Office of Environment and Heritage and the State of NSW are pleased to allow this material to be reproduced, for educational or non-commercial use, in whole or in part, provided the meaning is unchanged and its source, publisher and authorship are acknowledged. Specific permission is required for the reproduction of images. Disclaimer: The Office of Environment and Heritage NSW (OEH) has compiled this document in good faith, exercising all due care and attention. OEH does not accept responsibility for any inaccurate or incomplete information supplied by third parties. No representation is made about the accuracy, completeness or suitability of the information in this publication for any particular purpose. OEH shall not be liable for any damage which may occur to any person or organisation taking action or not on the basis of this publication. Readers should seek appropriate advice about the suitability of the information to their needs. Published by: Office of Environment and Heritage 59–61 Goulburn Street, Sydney PO Box A290 Sydney South NSW 1232 Report pollution and environmental incidents Environment Line: 131 555 (NSW only) or [email protected] See also www.environment.nsw.gov.au/pollution Ph: (02) 9995 5000 (switchboard) Ph: 131 555 (information & publications requests) Ph: 1300 361 967 (national parks, climate change and energy efficiency information and publications requests) Fax: (02) 9995 5999 TTY: (02) 9211 4723 Email: [email protected] Website: www.environment.nsw.gov.au ISBN 978 1 74359 743 9 OEH 2014/0636 August 2014 ii Integrated Regional Vulnerability Assessment: Riverina Murray CONTENTS Introduction .............................................................................................................. -

Business-Paper-July-2021.Pdf
Bland Shire Council Business Paper Ordinary Council Meeting 20 July 2021 i ETHICAL DECISION MAKING AND CONFLICTS OF INTEREST A guiding checklist for Councillors, Officers and Advisory Committees Ethical Decision Making • Is the decision or conduct legal? • Is it consistent with Government policy, Council’s objectives and Code of Conduct? • What will the outcome be for you, your colleagues, the Council, anyone else? • Does it raise a conflict of interest? • Could your possible conflict of interest lead to private gain or loss at public expense? • Can the decision be justified in terms of public interest? • Would it withstand public scrutiny? Conflict of Interest • A conflict of interest is a clash between private interest and public duty. There are two types of conflict: Pecuniary – regulated by the Local Government Act and Office of Local Government and, Non- pecuniary – regulated by Codes of Conduct and policy. ICAC, Ombudsman, Office of Local Government (advice only). The test for conflict of interest • Is it likely I could be influenced by personal interest in carrying out my public duty? • Would a fair and reasonable person believe I could be so influenced? • Conflict of interest is closely tied to the layperson’s definition of “corruption” – using public office for private gain. • Important to consider public perceptions of whether you have a conflict of interest Identifying Problems 1st - Do I have private interest affected by a matter I am officially involved in? 2nd - Is my official role one of influence or perceived influence over the matter? 3rd - Do my private interests conflict with my official role? Whilst seeking advice is generally useful, the ultimate decision rests with the person concerned. -

Cowal Overview
Cowal Overview Evolution Mining is a leading, growth focussed Australian gold company, forecasting to produce between 770,000oz and 820,000oz gold in FY16. Our company has continued to grow through acquisition, completing the acquisition of Cowal in July 2015 and Mungari in August 2015. Cowal gold operation is forecast to produce between 215,000oz and 240,000oz in FY16. Location: 350km west of Sydney Producing: Gold Site management: Jason Grieve - General Manager Mine Site contact number: +61 2 6975 4700 History Gold first NSW government Cowal Gold Poject is Construction Cowal Gold Operation is discovered in West issues sold to Homestake commences on granted extension of Wyalong area Development Mining Cowal Gold mine life through to 2024 Consent conditions Project for the Cowal Gold Project 1893 1988 1999 2000 2001 2002 2004 2006 2014 2015 North Limited Evolution undertakes extensive Homestake Mining Mining acquire exploration at Lake North Limited is merges with Barrick First gold is poured Cowal Gold Cowal acquired by Rio Tinto Gold in April 2006 Operation Geology The Cowal gold deposits (E41, E42, E46, Galway, and Regal) occur within the 40km long by 15km wide Ordovician Lake Cowal Volcanic Complex, east of the Gilmore Fault Zone within the eastern portion of the Lachlan Fold Belt. The overall structure of the gold deposits is complex but in general consists of a faulted antiform that plunges shallowly to the north-northeast. The deposits are aligned along a north-south orientated corridor with bounding faults, the Booberoi Fault on the western side and the Reflector Fault on the eastern side (the Gold Corridor). -

Riverina Region
A B C TO COBAR 134km D E Ck YATHONG Source: © DEPARTMENT OF LANDS NATURE B87 PANORAMA AVENUE BATHURST 2795 RESERVE www.lpi.nsw.gov.au BROKEN This map was produced and published by Cartoscope Pty Ltd and RANGE ST F KAJULIGAH may not be reproduced in any way without written permission of HHH WAY NAT RES the publishers. Properties bearing the stars symbols eg. YATHONG TO WILCANNIA 140km (the stars) are independently assessed by Star Ratings Australia, a ST F division of The Australian Tourism Industry Council Pty Ltd. The STARS are trademarks of Star Ratings Australia. For more 1 information about using star ratings go to www.starratings.com.au 1 CARTOSCOPE MAPSANDGUIDES Although great care has been taken, Cartoscope accepts no responsibility for any incident arising from any inaccuracy. Mount Hope We would appreciate notification of any errors. Address to Logontoseedetailed Cartoscope Pty Ltd, PO Box 1900, Hornsby Westfield NSW 1635 www.maps.com.au touringandholidaymaps, Editor: Louise Maggs. Cartography: Renate Rank. Computer graphics: Lauren Tabain & Terhi Ilander. NOMBINNIE informationandtopurchase Printed by Pegasus Print Group, Sydney. STATE Ivanhoe (locality) CONSERVATION mapsandguides. Railtown AREA (locality) ROUND HILL Conoble NAT RES (locality) Clickonthe 51 Trida weblinkbelow NOMBINNIE tologon 33º00'S NAT RES Roto OutbackNSW Map Fruit Fly Exclusion Zone 95 Foradjoining map seeCartoscope's www.maps.com.au B75 NOMBINNIE NATURE RESERVE KIDMAN Willandra MORRISONS (locality) LAKE NAT RES Lowlands 54 2 WILLANDRA NAT PK 2 Creek Grabamap... (locality) Mossgiel Fees apply andplanyournextgetaway Creek 46 78 52 www.lpi.nsw.gov.au Tourist drive - sealed surface, unsealed, highway................................. A1 20 Major road with km distance, secondary road................................... -

Bioregions of New South Wales – Their Biodiversity, Conservation and History
119 CHAPTER 10 The South Western Slopes Bioregion 1. Location 2. Climate Slopes Western South The NSW South Western Slopes Bioregion is an extensive area of foothills and This bioregion is dominated by a sub-humid climate characterised by hot isolated ranges comprising the lower inland slopes of the Great Dividing summers and no dry season. A temperate climate, with warm summers, Range extending from north of Cowra through southern NSW into western occurs at higher elevations along the eastern boundary of the bioregion Victoria with an area of 8,657,426 ha. About 8,070,608 ha or 93.22% of this adjacent to the South Eastern Highlands Bioregion. Mean annual bioregion occurs in NSW, with the remainder in Vic. (IBRA 5.1). The NSW temperature increases across the bioregion from low temperatures in the portion of the bioregion occupies about 10.1% of the state. south and east to higher temperatures in the north and west (Gibbons 2001). The bioregion is bounded by 6 other bioregions: the Riverina and Cobar Rainfall is distributed across the South Western Slopes Bioregion with high Peneplain bioregions to the west, Darling Riverine Plains and Brigalow Belt (up to around 1200mm) mean annual rainfall in the east, and lower values South bioregions to the north, Sydney Basin to the northeast and the South (around 400mm) for mean annual rainfall in the west (Gibbons 2001). Eastern Highlands Bioregion running along much of the eastern boundary. The bioregion extends from Albury in the south to Dunedoo in the northeast. Towns located in the bioregion include Wagga Wagga, Mudgee, Cootamundra, Narrandera, Parkes, Gundagai and Young. -
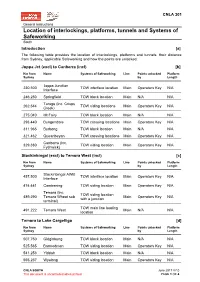
Location of Interlockings, Platforms, Tunnels and Systems of Safeworking South
CNLA 301 General instructions Location of interlockings, platforms, tunnels and Systems of Safeworking South Introduction [a] The following table provides the location of interlockings, platforms and tunnels, their distance from Sydney, applicable Safeworking and how the points are unlocked. Joppa Jct (excl) to Canberra (incl) [b] Km from Name Systems of Safeworking Line Points unlocked Platform Sydney by Length Joppa Junction 230.500 TOW interface location Main Operators Key N/A Interface 246.250 Springfield TOW block location Main N/A N/A Tarago (Inc. Crisps 262.544 TOW siding locations Main Operators Key N/A Creek) 275.040 Mt Fairy TOW block location Main N/A N/A 293.440 Bungendore TOW crossing locations Main Operators Key N/A 311.965 Burbong TOW block location Main N/A N/A 321.462 Queanbeyan TOW crossing locations Main Operators Key N/A Canberra (Inc. 329.380 TOW siding location Main Operators Key N/A Fyshwick) Stockinbingal (excl) to Temora West (incl) [c] Km from Name Systems of Safeworking Line Points unlocked Platform Sydney by Length Stockinbingal AWB 457.500 TOW interface location Main Operators Key N/A Interface 474.441 Combaning TOW siding location Main Operators Key N/A Temora (Inc. TOW siding location 489.290 Temora Wheat sub Main Operators Key N/A with a junction terminal) TOW main line loading 491.222 Temora West Main N/A N/A location Temora to Lake Cargelligo [d] Km from Name Systems of Safeworking Line Points unlocked Platform Sydney by Length 507.750 Gidginbung TOW block location Main N/A N/A 525.565 Barmedman TOW siding -

MAP 146-32 12 20 HWY 144 -29 14 15 148-29 146º Babinda 147º 71 148º 15 26 32º Yarrania 32 Bogan 32º 14 32 Trangie MITCHELL 24 Nymagee
18 Kidman A B CDCathundral Gin Gin JOINS 16 JOINS 146-29 Buddabaddah 18 JOINS MAP 146-32 12 20 HWY 144 -29 14 15 148-29 146º Babinda 147º 71 148º 15 26 32º Yarrania 32 Bogan 32º 14 32 Trangie MITCHELL 24 Nymagee Yarranvale Bulbodney 40 Five Ways 42 Mungeribar 40 48 Tabratong 34 31 32 18 48 ange P Way Glenwood Lansdale 19 1 37 Tottenham Dandaloo 1 Bombah 57 Nangerybone 13 Bobadah River 14 Mogal Creek 27 Bedooba Plain Albert Farrendale Walkers 13 44 Gilgunnia Hill Tinda 46 13 Ballatta Kerriwah Eremaran V Terowie 256 31 30 Yellow Mungery Creek Mountain Burthong 53 Yethera 40 26 an 30 Warge Rock 19 39 Vermont Kidm Boona Tullamore Kerein Hills Mountains Bulgandramine 40 15 Eribung 15 8 17 34 Melrose 14 V Tallebung 23 Dunmore Bogolo Mt Tallebung Kadungle 2 14 36 15 2 66 22 Ghost Woggoon Hill V Fifield V Mount Hope Flamingo Nature Reserve Boor Hill 51 30 60 Tr undle 15 28 22 25 Round Hill 31 31 33 Matakana V 30 35 28 18 Mt Tilga 34 Blowclear 33º Gunebang V Euabalong Kiacatoo Byong West V Hills 33º Condobolin Nanardine 10 23 Derriwong Bogan 12 11 19 23 Gate Round Hill Euabalong Gunningbland 42 Nature Reserve 43 Ootha 108 16 Yarrabandai 13 Brolgan Murrin Bridge Wallaroi V Lachlan 28 Hill 30 Warregal 87 14 37 Tichborne 3 17 3 24 Banar Corridgery Lake Cargelligo Lake Lake 36 Fairholme 21 Cargelligo 7 27 Bogandillan Warroo HWY 22 Wargambegal Bedgerebong 8 V Lake Swamp 13 Forbes Mt Bowen Burgooney 106 15 River 93 23 Tullibigeal 36 Corinella 17 Nerang 15 Way Lake Cargelligo Canal 37 39 Brewster 35 117 24 Bena 8 Bundaburrah 47 Weja Burcher Garema New