Microinjection of Actin-Binding Proteins and Actin Antibodies Demonstrates Involvement of Nuclear Actin in Transcription of Lampbrush Chromosomes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

On the Positions of Centromeres in Chicken Lampbrush Chromosomes
Chromosome Research (2006) 14:777–789 # Springer 2006 DOI: 10.1007/s10577-006-1085-y On the positions of centromeres in chicken lampbrush chromosomes Alla Krasikova, Svetlana Deryusheva, Svetlana Galkina, Anna Kurganova, Andrei Evteev & Elena Gaginskaya* Biological Research Institute, Saint-Petersburg State University, Oranienbaumskoie sch. 2, Stary Peterhof, Saint-Petersburg, 198504, Russia; Tel: +7-812-4507311; Fax: +7-812-4507310; E-mail: [email protected] *Correspondence Received 5 May 2006. Received in revised form and accepted for publication by Mary Delany 24 July 2006 Key words: centromere, chicken, CNM, cohesin, lampbrush chromosome Abstract Using immunostaining with antibodies against cohesin subunits, we show here that cohesin-enriched structures analogous to the so-called centromere protein bodies (PB) are the characteristic of galliform lampbrush chromosomes. Their centromeric location was verified by FISH with certain DNA probes. PB-like structures were used as markers for centromere localization in chicken lampbrush chromosomes. The gap predicted to be centromeric in current chicken chromosome 3 sequence assembly was found to correspond to the non- centromeric cluster of CNM repeat on the q-arm of chromosome 3; the centromere is proposed to be placed at another position. The majority of chicken microchromosomes were found to be acrocentric, in contrast to Japanese quail microchromosomes which are biarmed. Centromere cohesin-enriched structures on chicken and quail lampbrush microchromosomes co-localize with pericentromeric CNM and BglIIj repeats respectively. FISH to the nascent transcripts on chicken lampbrush chromosomes revealed numerous non-centromeric CNM clusters in addition to pericentromeric arrays. Complementary CNM transcripts from both C- and G-rich DNA strands were revealed during the lampbrush stage. -
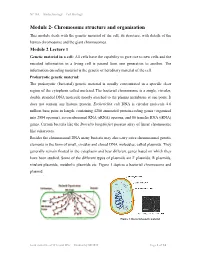
Chromosome Structure and Organisation
NPTEL – Biotechnology – Cell Biology Module 2- Chromosome structure and organisation This module deals with the genetic material of the cell, its structure, with details of the human chromosome and the giant chromosomes. Module 2 Lecture 1 Genetic material in a cell: All cells have the capability to give rise to new cells and the encoded information in a living cell is passed from one generation to another. The information encoding material is the genetic or hereditary material of the cell. Prokaryotic genetic material: The prokaryotic (bacterial) genetic material is usually concentrated in a specific clear region of the cytoplasm called nucleiod. The bacterial chromosome is a single, circular, double stranded DNA molecule mostly attached to the plasma membrane at one point. It does not contain any histone protein. Escherichia coli DNA is circular molecule 4.6 million base pairs in length, containing 4288 annotated protein-coding genes (organized into 2584 operons), seven ribosomal RNA (rRNA) operons, and 86 transfer RNA (tRNA) genes. Certain bacteria like the Borrelia burgdorferi possess array of linear chromosome like eukaryotes. Besides the chromosomal DNA many bacteria may also carry extra chromosomal genetic elements in the form of small, circular and closed DNA molecules, called plasmids. They generally remain floated in the cytoplasm and bear different genes based on which they have been studied. Some of the different types of plasmids are F plasmids, R plasmids, virulent plasmids, metabolic plasmids etc. Figure 1 depicts a bacterial chromosome and plasmid. Figure 1: Bacterial genetic material Joint initiative of IITs and IISc – Funded by MHRD Page 1 of 24 NPTEL – Biotechnology – Cell Biology Virus genetic material: The chromosomal material of viruses is DNA or RNA which adopts different structures. -

Comparison of Active Transcription Regions of Lampbrush Chromosomes with the Mitotic Chromosome G Pattern in the European Domestic Goose Anser Anser
Archiv Tierzucht 54 (2011) 1, 69-82, ISSN 0003-9438 69 © Leibniz Institute for Farm Animal Biology, Dummerstorf, Germany Comparison of active transcription regions of lampbrush chromosomes with the mitotic chromosome G pattern in the European domestic goose Anser anser Katarzyna Andraszek and Elżbieta Smalec Department of Animal Genetics and Horse Breeding, Institute of Bioengineering and Animal Breeding, University of Life Sciences and Humanities, Poland Abstract The most complete information on the karyotype is acquired through the observation of chromosomes obtained from dividing cells. A high number of chromosomes and the presence of microchromosomes in the bird karyotype have made cytogeneticists look for other sources of information on chromosomes. Information sources of great value for the bird karyotype analysis are meiotic chromosomes, specifically represented by lampbrush chromosomes. Lampbrush chromosomes (LBCs) found in developing oocytes of birds are perceived as a new model in cytogenetics which is especially important in the analysis of bird chromosomes. A typical LBC analysis enables one to assess transcription activity on the basis of LBC morphology (inactive chromomeres and side loops). A comparison of lampbrush chromosome transcription activity and the GTG pattern of the corresponding mitotic chromosomes have proven that active transcription regions with side loops correspond to G-positive bands on mitotic chromosomes. Keywords: geese, Anser anser, lampbrush chromosomes (LBCs), mitotic chromosomes, transcription, G-bands Zusammenfassung Vergleich zwischen den transkriptionell aktiven Regionen der Lampenbürstenchromosomen und dem G-Muster der mitotischen Chromosomen der Europäischen Hausgans Anser anser Die vollständigsten Informationen über den Karyotyp erhält man durch die Beobachtung von Chromosomen sich teilendender Zellen. Die große Anzahl von Chromosomen und das Vorhandensein von Mikrochromosomen im Vogelkaryotyp haben Zytogenetiker zum Suchen von anderen Informationsquellen über Chromosomen veranlasst. -

1 Are Lampbrush Chromosomes Unique to Meiotic Cells? Joseph G
1 Are lampbrush chromosomes unique to meiotic cells? Joseph G. Gall Department of Embryology, Carnegie Institution for Science, Baltimore MD 21218 Corresponding author: Joseph G. Gall Department of Embryology Carnegie Institution for Science 3520 San Martin Drive Baltimore, MD 21218 Phone: (410) 246-3017 e-mail: [email protected] Running title: Lampbrush chromosomes and meiosis Key words: lampbrush chromosome, meiosis, oocyte, oogenesis, polyploidy, reprogramming, transcription. Abbreviations: GV, germinal vesicle; LBC, lampbrush chromosome; pol II, polymerase II; TU, transcription unit. 2 Abstract Lampbrush chromosomes (LBCs) are transcriptionally active chromosomes found in the germinal vesicle (GV) of large oocytes of many vertebrate and invertebrate animals and also in the giant single-celled alga Acetabularia. These cells are all in prophase of the first meiotic division. Nevertheless, many meiotic cells do not develop LBCs, arguing that LBCs are not an essential feature of meiosis. LBCs probably represent the most active transcriptional state that can be attained by cells that must give rise to diploid progeny. Polyploidy permits cells to reach higher rates of transcription per nucleus but precludes a return to diploidy. In this sense LBCs represent a relatively inefficient transcriptional compromise employed by large meiotic cells. These considerations help to explain why transcriptionally active GVs develop LBCs, but they do not explain why LBCs have never been seen in somatic cells, diploid or otherwise. If LBCs are truly limited to germ cells, then some of their unusual features may reflect reprogramming of the genome. If this is the case, LBCs provide unique opportunities to study reprogramming at the level of the individual transcription unit. -

Structure of Lampbrush Chromosome Loops During Different States of Transcriptional Activity As Visualized in the Presence
Biology of the Cell, 59 (1987) 33-42 33 © Elsevier, Paris Original article Structure of lampbrush chromosome loops during different states of transcriptional activity as visualized in the presence I of physiological salt concentrations l,. Ulrich SCHEER * Division of Membrane Biology and Biochemistry, Institute of Cell and Tumor Biology, German Cancer Research Center, D-6900 Heidelberg, F.R.G. (Received 11-7-1986; accepted 17-11-1986) Lampbrush chromosomes of amphibian oocytes were isolated in the presence of near-physiological salt concentrations, to preserve their native state, and studied by electron microscopy of ultrathin s~dions. The transcriptional state of the lampbrush chromosomes was experimentally modulated by incubating the oocytes for various time periods in medium containing actinomycin D. The observations show that the structure of the lateral loops changes rapidly in response to alterations in transcriptional activity. During decreasing transcriptional activity and reduced packing density of transcripts, the chromatin axis first condensed into nucleosomes and then into an approximately 30 nm thick higher order chromatin fiber. Packaging of the loop axis into supranucleosomal structures may contribute to the foreshortening and retraction of the loops observed during inhibition of transcription and in later stages of meiotic prophase. The increasing packing density of the DNA during the retraction process of the loops could also be visualized by immunofluorescence microscopy using antibodies to DNA. The dependence of the loop chromatin structure on transcriptional activity is discussed in relation to current views of mechanisms involved in gene activation. lampbrush chromosomes - chromatin structure - electron microscopy - immunofluorescence microscopy - DNA antibodies INTRODUCTION transcribing RN A polymerases the chromatin fiber is largely extended and nucleosomes are absent [13]. -

Amphibian and Avian Karyotype Evolution: Insights from Lampbrush Chromosome Studies
G C A T T A C G G C A T genes Review Amphibian and Avian Karyotype Evolution: Insights from Lampbrush Chromosome Studies Anna Zlotina †, Dmitry Dedukh † and Alla Krasikova * Saint-Petersburg State University, Saint-Petersburg 199034, Russia; [email protected] (A.Z.); [email protected] (D.D.) * Correspondence: [email protected]; Tel.: +7-812-328-96-87 † These authors contributed equally to this work. Received: 15 September 2017; Accepted: 31 October 2017; Published: 8 November 2017 Abstract: Amphibian and bird karyotypes typically have a complex organization, which makes them difficult for standard cytogenetic analysis. That is, amphibian chromosomes are generally large, enriched with repetitive elements, and characterized by the absence of informative banding patterns. The majority of avian karyotypes comprise a small number of relatively large macrochromosomes and numerous tiny morphologically undistinguishable microchromosomes. A good progress in investigation of amphibian and avian chromosome evolution became possible with the usage of giant lampbrush chromosomes typical for growing oocytes. Due to the giant size, peculiarities of organization and enrichment with cytological markers, lampbrush chromosomes can serve as an opportune model for comprehensive high-resolution cytogenetic and cytological investigations. Here, we review the main findings on chromosome evolution in amphibians and birds that were obtained using lampbrush chromosomes. In particular, we discuss the data on evolutionary chromosomal rearrangements, accumulation of polymorphisms, evolution of sex chromosomes as well as chromosomal changes during clonal reproduction of interspecies hybrids. Keywords: lampbrush chromosomes; chromosomal evolution; amphibians; birds; karyotype; sex chromosomes 1. Peculiarities of Amphibian and Avian Genomes and Karyotypes In general, amphibian and avian species are characterized by specific and complexly organized genomes. -

Germline-Restricted Chromosome (GRC) Is Widespread Among Songbirds
Germline-restricted chromosome (GRC) is widespread among songbirds Anna A. Torgashevaa,b, Lyubov P. Malinovskayaa,b, Kira S. Zadesenetsa, Tatyana V. Karamyshevaa, Elena A. Kizilovaa,b, Ekaterina A. Akberdinaa, Inna E. Pristyazhnyuka, Elena P. Shnaiderc, Valeria A. Volodkinad, Alsu F. Saifitdinovad, Svetlana A. Galkinad, Denis M. Larkina,e,1, Nikolai B. Rubtsova,b, and Pavel M. Borodina,b,1 aInstitute of Cytology and Genetics, Russian Academy of Sciences, Siberian Department, 630090 Novosibirsk, Russia; bDepartment of Cytology and Genetics, Novosibirsk State University, 630090 Novosibirsk, Russia; cSibEcoCenter LLC, 630009 Novosibirsk, Russia; dDepartment of Genetics and Biotechnology, Saint Petersburg State University, 199034 Saint Petersburg, Russia; and eDepartment of Comparative Biomedical Sciences, Royal Veterinary College, University of London, NW1 0TU London, United Kingdom Edited by Scott V. Edwards, Harvard University, Cambridge, MA, and approved April 8, 2019 (received for review November 15, 2018) An unusual supernumerary chromosome has been reported for sequences, together with the zebra finch and chicken somatic cell two related avian species, the zebra and Bengalese finches. This counterparts, suggests that the GRC was formed after the galli- large, germline-restricted chromosome (GRC) is eliminated from form–neoaves split (9). A recent study hypothesized that the GRC somatic cells and spermatids and transmitted via oocytes only. Its is evolutionarily old and could be present in other birds as well (7). origin, distribution among avian lineages, and function were To answer the questions about the origin, architecture, and dis- mostly unknown so far. Using immunolocalization of key meiotic tribution of GRCs in avian lineages, we performed a comprehensive proteins, we found that GRCs of varying size and genetic content comparative cytogenetic study of the germ cell chromosomes from are present in all 16 songbird species investigated and absent from 24 avian species representing eight orders. -

Cytological Maps of Lampbrush Chromosomes of European Water
Dedukh et al. BMC Genetics 2013, 14:26 http://www.biomedcentral.com/1471-2156/14/26 RESEARCH ARTICLE Open Access Cytological maps of lampbrush chromosomes of European water frogs (Pelophylax esculentus complex) from the Eastern Ukraine Dmitry Dedukh1, Glib Mazepa2,3, Dmitry Shabanov3, Juriy Rosanov4, Spartak Litvinchuk4, Leo Borkin5, Alsu Saifitdinova1 and Alla Krasikova1* Abstract Background: Hybridogenesis (hemiclonal inheritance) is a kind of clonal reproduction in which hybrids between parental species are reproduced by crossing with one of the parental species. European water frogs (Pelophylax esculentus complex) represent an appropriate model for studying interspecies hybridization, processes of hemiclonal inheritance and polyploidization. P. esculentus complex consists of two parental species, P. ridibundus (the lake frog) and P. lessonae (the pool frog), and their hybridogenetic hybrid – P. esculentus (the edible frog). Parental and hybrid frogs can reproduce syntopically and form hemiclonal population systems. For studying mechanisms underlying the maintenance of water frog population systems it is required to characterize the karyotypes transmitted in gametes of parental and different hybrid animals of both sexes. Results: In order to obtain an instrument for characterization of oocyte karyotypes in hybrid female frogs, we constructed cytological maps of lampbrush chromosomes from oocytes of both parental species originating in Eastern Ukraine. We further identified certain molecular components of chromosomal marker structures and mapped coilin-rich spheres and granules, chromosome associated nucleoli and special loops accumulating splicing factors. We recorded the dissimilarities between P. ridibundus and P. lessonae lampbrush chromosomes in the length of orthologous chromosomes, number and location of marker structures and interstitial (TTAGGG)n-repeat sites as well as activity of nucleolus organizer. -

Avian Lampbrush Chromosomes: a Powerful Tool for Exploration of Genome Expression
Review Article Cytogenet Genome Res 2009;124:251–267 Accepted after revision: October 14, 2008 DOI: 10.1159/000218130 by A. Houben Avian Lampbrush Chromosomes: a Powerful Tool for Exploration of Genome Expression E. Gaginskaya T. Kulikova A. Krasikova Saint-Petersburg State University, Saint-Petersburg, Russia Key Words some specific features: very long transcription units, deregu- Chicken genome ؒ C h r o m o s o m e o r g a n i z a t i o n ؒ L a m p b r u s h lated termination, and transcription of non-coding satellite chromosomes ؒ Non-coding RNA ؒ Oocyte nucleus ؒ repeats. Here, based on the modern view on a role of RNA ,Oogenesis ؒ T r a n s c r i p t i o n interference machinery in regulation of genome expression we suggest a mechanism of initiation of satellite DNA tran- scription and offer a novel interpretation of the ‘classical’ hy- Abstract pothesis that sought to explain the significance of wide- Lampbrush chromosomes (LBCs) are highly extended biva- spread transcription during oocyte growth. lents that function in the growing oocytes of many animals. Copyright © 2009 S. Karger AG, Basel Due to their distinctive chromomere-loop organization and intense transcriptional activity of lateral loops the LBCs, mainly amphibian ones, have served as a powerful system The investigation of genome structure and function for exploring the general principles of chromosome organi- has been greatly helped by studies of model systems such zation and function. The exploitation of avian LBCs has con- as polytene and lampbrush chromosomes that facilitate siderably broadened the opportunities for comparative exploration of gene activity in vivo. -

Structure and Functions of Lampbrush Chromosomes
BioTechnologia vol. 92(4) C pp. 337-344 C 2011 Journal of Biotechnology, Computational Biology and Bionanotechnology REVIEW PAPER Structure and functions of lampbrush chromosomes KATARZYNA ANDRASZEK *, ELŻBIETA SMALEC Department of Animal Genetics and Horse Breeding, Siedlce University of Natural Sciences and Humanities, Siedlce, Poland * Corresponding author: [email protected] Abstract Lampbrush Chromosomes (LBCs) are present in the oocytes of birds, lower vertebrata and invertebrates during the prolonged prophase of the first meiotic division. Their name stems from their similarity to bottle brushes. Lampbrush chromosome of the early prophase is a bivalent, made up of two conjugating homologues. The axis of each homologous chromosome is formed by sister chromatids that are differentiated into regions of transcrip- tionally active and inactive chromatin. Transcription activity of LBCs is observed as a mantle of symmetrically distributed side loops along the chromosome axis. Changes in transcriptional activity are reflected in changes in their morphology. Transcriptional activity of LBCs is directly connected with physiological processes of the body and shows in the morphological structure of the chromosomes. The use of cytogenetic techniques and in situ hybridization have made it possible to identify unique and repeating sequences as well as DNA replication pro- teins in LBCs. Particularly, interesting prospects are offered by the possibility of using LBCs in studies of trans- criptional activity, cytogenetic investigations of karyotype evolution and genome mapping. Key words: meiotic chromosome, lampbrush chromosome, transcription The progress in biotechnology that was witnessed at Oocyte development takes place during the meiotic the turn of 20th and 21st century was a breakthrough prophase and for the majority of species ends in in scientific research – predominantly of experimental the metaphase of the first meiotic division. -
120224-FT Fribourg 06 Slides
Chromatin Structure and Function Fribourg 120224 -120316 Chromatin Structure and Function Handouts in Transcription, Replication, Repair 120224 1615 -1900 Fritz Thoma Institute of Molecular Health Science (previous Institute of Cell Biology) ETH-Zürich Hönggerberg HPM-E42 +41-44-6333323 [email protected] http://www.cell.biol.ethz.ch/research/thoma/ FT Fribourg12F 2 FT Fribourg12F 1 Chromatin - Organization Structural and Functional Heterogeneity of Chromatin Structures Mechanisms dynamic Nuclear Compartments Transcription Foci & Factories Replication Chromosome Territories Recombination Heterochromatin DNA-Repair Euchromatin Chromatin Controls Specialized Chromatin Loops DNA Gene Control Regions of Chromatin Promoters Fibers (30nm) Origins of Replication Physiological Centromeres Telomeres dynamic Remodelling Complexes Low Salt H2B H2A H2B Nucleosome H3 Filament RNAP H2A H4 H2B Histone-Variants Non-Histone- Histones: Histone-Modifications Chromosomal H1 „Histone Code“ Proteins 2x(H2A, H2B, H3, H4) FT Fribourg12F Lodish et al 5th ed 3 FT Fribourg12F 4 Chromatin Structures Locus Specific Heterogeneity in Structure and Function Genome-wide patterns of histone modifications in yeast. Millar, C.B., and Grunstein, M. (2006). Nat Rev Mol Cell Biol 7, 657-666. FT Fribourg12F 5 FT Fribourg12F 6 Chromatin Structure and Function Chromatin Structure and Function Focus Chromatin ? Chromatin Structures Transcription Replication Repair Recombination Aims Concepts - Facts – Fiction Terms, Keywords Key Experiments Approaches Methods "Feeling for -
Modifications of Lampbrush Chromosome Structure of the European Domestic Goose Anser Anser
Archiv Tierzucht 55 (2012) 1, 84-96, ISSN 0003-9438 © Leibniz Institute for Farm Animal Biology, Dummerstorf, Germany Modifications of lampbrush chromosome structure of the European domestic goose Anser anser Katarzyna Andraszek and Elżbieta Smalec Department of Animal Genetics and Horse Breeding, Institute of Bioengineering and Animal Breeding, University of Life Sciences and Humanities, Siedlce, Poland Abstract Lampbrush chromosomes (LBCs) represent a new model in avian cytogenetics and are increasingly more often used in poultry chromosome analyses. Additionally, lampbrush chromosomes are considered as model structures in the study of transcription regulation. Changes in transcription activity are reflected as modifications of LBC morphological structure and associated with physiological processes in the organism. The aim of the present study was to compare transcriptional activity of the first five lampbrush macrochromosomes and ZW sex lampbrush bivalents sampled from the oocytes of geese prior to and after the reproductive period. The respective bivalents sampled before and after reproduction have similar sizes but differ in morphological structure. Side loops of lampbrush chromosomes are sites of transcription activity. The activity varies according to the loop size. As the loops become more prominent, the activity grows and vice versa. Lampbrush chromosomes sampled after reproduction have smaller side loops. On the other hand, inactive chromomeres become prominent in the chromosomes. Marker loops are the last structures to be degraded after the end of reproduction. Consequently, they are used for identifying particular bivalents at different stages of cellular transcriptional activity. Keywords: Anser anser goose, reproduction, meiotic chromosome, lampbrush chromosome, transcription Introduction High transcriptional activity observed in avian oocytes during previtellogenesis causes chromatin decondensation.