Lecture 3 Chromosome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chloroplast Transit Peptides: Structure, Function and Evolution
reviews Chloroplast transit Although the first demonstration of precursor trans- port into chloroplasts was shown over two decades peptides: structure, ago3,4, only now is this area of cell biology becom- ing well understood. Many excellent reviews have been published recently on the evolution of plas- function and tids5, the evolution of organelle genomes6, the mechanism of gene transfer from organelles to the nucleus7 and the mechanism of protein import into evolution chloroplasts8,9. Proteins destined to plastids and other organ- elles share in common the requirement for ‘new’ Barry D. Bruce sequence information to facilitate their correct trafficking within the cell. Although in most cases this information resides in a cleavable, N-terminal sequence often collectively referred to as signal It is thought that two to three thousand different proteins are sequence, the different organelle-targeting se- targeted to the chloroplast, and the ‘transit peptides’ that act as quences have distinct properties and names: ‘signal peptides’ for the endoplasmic reticulum, chloroplast targeting sequences are probably the largest class of ‘presequences’ for the mitochondria and ‘transit peptides’ for chloroplasts and other plastids. This targeting sequences in plants. At a primary structural level, transit review focuses on recent progress in dissecting peptide sequences are highly divergent in length, composition and the role of the stromal-targeting domain of chloro- plast transit peptides. I will consider briefly the organization. An emerging concept suggests that transit peptides multitude of distinct functions that transit peptides contain multiple domains that provide either distinct or overlapping perform, provide an update on the limited struc- tural information of a number of transit peptides functions. -

Centrosome-Nuclear Envelope Tethering and Microtubule Motor
bioRxiv preprint doi: https://doi.org/10.1101/442368; this version posted October 12, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Centrosome-nuclear envelope tethering and microtubule motor-based pulling forces collaborate in centrosome positioning during mitotic entry Vincent Boudreau1, Richard Chen1, Alan Edwards1, Muhammad Sulaimain1, Paul S. Maddox1* 1 Department of Biology, University of North Carolina at Chapel Hill * Direct all correspondence to this author at [email protected]. Centrosome positioning relative to the nucleus and cell nuclear envelope and at the cortex to ensure proper centrosome shape is highly regulated across cell types, during cell migration positioning (De Simone et al., 2016). Dynein regulators including and during spindle formation in cell division. Across most sexual- LIS-1 are also required for centrosome separation in the embryo ly reproducing animals, centrosomes are provided to the oocyte (Cockell et al., 2004), although their spatio-temporal contributions through fertilization and must be positioned properly to establish remain elusive. Despite our understanding of microtubule cyto- the zygotic mitotic spindle. How centrosomes are positioned in skeleton-based pulling forces, the contribution of key mitotic kinas- space and time through the concerted action of key mitotic en- es and phosphatases to centrosome positioning remains unclear. try biochemical regulators including Protein Phosphatase 2A The net effect of molecular regulators is a biophysical (PP2A-B55/SUR-6), biophysical regulators including Dynein mechanism required for positioning centrosomes during mitotic and the nuclear lamina is unclear. -

Introduction to the Cell Cell History Cell Structures and Functions
Introduction to the cell cell history cell structures and functions CK-12 Foundation December 16, 2009 CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and distribution of high quality educational content that will serve both as core text as well as provide an adaptive environment for learning. Copyright ©2009 CK-12 Foundation This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License. To view a copy of this license, visit http://creativecommons.org/licenses/by-sa/3.0/us/ or send a letter to Creative Commons, 171 Second Street, Suite 300, San Francisco, California, 94105, USA. Contents 1 Cell structure and function dec 16 5 1.1 Lesson 3.1: Introduction to Cells .................................. 5 3 www.ck12.org www.ck12.org 4 Chapter 1 Cell structure and function dec 16 1.1 Lesson 3.1: Introduction to Cells Lesson Objectives • Identify the scientists that first observed cells. • Outline the importance of microscopes in the discovery of cells. • Summarize what the cell theory proposes. • Identify the limitations on cell size. • Identify the four parts common to all cells. • Compare prokaryotic and eukaryotic cells. Introduction Knowing the make up of cells and how cells work is necessary to all of the biological sciences. Learning about the similarities and differences between cell types is particularly important to the fields of cell biology and molecular biology. -

The Nature of Genomes Viral Genomes Prokaryotic Genome
The nature of genomes • Genomics: study of structure and function of genomes • Genome size – variable, by orders of magnitude – number of genes roughly proportional to genome size • Plasmids – symbiotic DNA molecules, not essential – mostly circular in prokaryotes • Organellar DNA – chloroplast, mitochondrion – derived by endosymbiosis from bacterial ancestors Chapter 2: Genes and genomes © 2002 by W. H. Freeman and Company Chapter 2: Genes and genomes © 2002 by W. H. Freeman and Company Viral genomes • Nonliving particle In prokaryotes, viruses are – nucleic acid sometimes referred to as – protein bacteriophages. • DNA or RNA – single-stranded or double-stranded – linear or circular • Compact genomes with little spacer DNA Chapter 2: Genes and genomes © 2002 by W. H. Freeman and Company Chapter 2: Genes and genomes © 2002 by W. H. Freeman and Company Prokaryotic genome • Usually circular double helix – occupies nucleoid region of cell – attached to plasma membrane • Genes are close together with little intergenic spacer • Operon – tandem cluster of coordinately regulated genes – transcribed as single mRNA • Introns very rare Chapter 2: Genes and genomes © 2002 by W. H. Freeman and Company Chapter 2: Genes and genomes © 2002 by W. H. Freeman and Company 1 Eukaryotic nuclear genomes • Each species has characteristic chromosome number • Genes are segments of nuclear chromosomes • Ploidy refers to number of complete sets of chromosomes –haploid (1n): one complete set of genes – diploid (2n) – polyploid (≥3n) • In diploids, chromosomes come in homologous pairs (homologs) In humans, somatic cells have – structurally similar 2n = 46 chromosomes. – same sequence of genes – may contain different alleles Chapter 2: Genes and genomes © 2002 by W. H. -

Principles of Human Anatomy
Principles of Cells Human Anatomy Cells are the basic living structural, Eleventh Edition functional unit of the body Gerard J. Tortora Cytology is the branch of science that & studies cells Mark T. Nielsen The human body has 100 trillion cells 200 CHAPTER 2 different cell types with a variety of Cells shapes, sizes and functions. Copyright © 2007 by John Wiley & Sons, Inc. Cell Diversity Generalized Cell Sizes (diameter) Ovum – 140 µm RBC – 8 µm Major parts of a cell µm = 1/10,000 of a cm Shapes Plasma membrane Flat Cytoplasm Oval Cubed Organelles Star shaped Elongated Inclusions Concave Structures Flagella Cilia Microvilli Fluid mosaic model of the plasma membrane Membrane Lipids Phospholipids – 75% Lipid bilayer Glycolipids – 5% Self recognition Cholesterol – 20% Maintains integrity Maintains fluidity Membrane Proteins Functions of the Cell Membrane Integral proteins Extend across the Communication phospholipid bilayer Shape & protection Channels Pores Maintains the electrochemical gradient Receptors Electrical separation of charge Transporters Enzymes Chemical (concentration gradient) Peripheral proteins Selective permeability Loosely attached to inner or outer surface Some substances easily travel across the Enzymes membrane and others do not Cytoskeletal anchors Membrane Transport Membrane Transport Active transport (uses ATP) Passive transport (kinetic energy not ATP) Primary active transport Net diffusion Molecule mover hydrolyzes ATP Movement of molecules from [high] to [low] -

The Recognition and Mobility of DNA Double-Strand Breaks
The Recognition and Mobility of DNA Double-Strand Breaks by Jonathan Strecker A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Graduate Department of Molecular Genetics University of Toronto © Copyright by Jonathan Strecker 2016 The Recognition and Mobility of DNA Double-Strand Breaks Jonathan Strecker Doctor of Philosophy Graduate Department of Molecular Genetics University of Toronto 2016 Abstract DNA double-strand breaks (DSBs) pose a threat to cell survival and genomic integrity, and remarkable mechanisms exist to deal with these breaks. A single DSB activates a signalling response that profoundly impacts cell physiology, not least through the engagement of DSB repair pathways and the arrest of cell division. Here I study these processes in the budding yeast Saccharomyces cerevisiae and investigate two central themes to this response. First, I examine how the natural ends of chromosomes, telomeres, are differentiated from DSBs by generating DNA ends with increasing telomeric character. I discover a striking transition in the activity of the telomerase inhibitor Pif1 at these ends and propose that this is the dividing line between DSBs and telomeres. Second, I investigate a phenomenon whereby a DSB increases the mobility of chromosomes within the nucleus. This increase in mobility is dependent on the Mec1 kinase and is proposed to promote repair by homologous recombination. I identify that the Mec1-dependent phosphorylation of Cep3, a kinetochore component, is required to stimulate chromatin mobility following DNA breakage and provide a new model for how a DSB affects the constraints on chromosomes. Unexpectedly, I find that increased mobility is not required for DSB repair and instead propose that Cep3 helps arrest the cell cycle in response to a DSB. -

Centrosome Positioning in Vertebrate Development
Commentary 4951 Centrosome positioning in vertebrate development Nan Tang1,2,*,` and Wallace F. Marshall2,` 1Department of Anatomy, Cardiovascular Research Institute, The University of California, San Francisco, USA 2Department Biochemistry and Biophysics, The University of California, San Francisco, USA *Present address: National Institute of Biological Science, Beijing, China `Authors for correspondence ([email protected]; [email protected]) Journal of Cell Science 125, 4951–4961 ß 2012. Published by The Company of Biologists Ltd doi: 10.1242/jcs.038083 Summary The centrosome, a major organizer of microtubules, has important functions in regulating cell shape, polarity, cilia formation and intracellular transport as well as the position of cellular structures, including the mitotic spindle. By means of these activities, centrosomes have important roles during animal development by regulating polarized cell behaviors, such as cell migration or neurite outgrowth, as well as mitotic spindle orientation. In recent years, the pace of discovery regarding the structure and composition of centrosomes has continuously accelerated. At the same time, functional studies have revealed the importance of centrosomes in controlling both morphogenesis and cell fate decision during tissue and organ development. Here, we review examples of centrosome and centriole positioning with a particular emphasis on vertebrate developmental systems, and discuss the roles of centrosome positioning, the cues that determine positioning and the mechanisms by which centrosomes respond to these cues. The studies reviewed here suggest that centrosome functions extend to the development of tissues and organs in vertebrates. Key words: Centrosome, Development, Mitotic spindle orientation Introduction radiating out to the cell cortex (Fig. 2A). In some cases, the The centrosome of animal cells (Fig. -
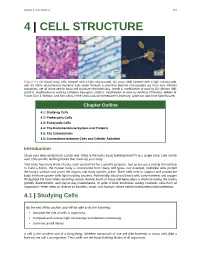
Chapter 4 – Cell Structure
Chapter 4 | Cell Structure 107 4 | CELL STRUCTURE Figure 4.1 (a) Nasal sinus cells (viewed with a light microscope), (b) onion cells (viewed with a light microscope), and (c) Vibrio tasmaniensis bacterial cells (seen through a scanning electron microscope) are from very different organisms, yet all share certain basic cell structure characteristics. (credit a: modification of work by Ed Uthman, MD; credit b: modification of work by Umberto Salvagnin; credit c: modification of work by Anthony D'Onofrio, William H. Fowle, Eric J. Stewart, and Kim Lewis of the Lewis Lab at Northeastern University; scale-bar data from Matt Russell) Chapter Outline 4.1: Studying Cells 4.2: Prokaryotic Cells 4.3: Eukaryotic Cells 4.4: The Endomembrane System and Proteins 4.5: The Cytoskeleton 4.6: Connections between Cells and Cellular Activities Introduction Close your eyes and picture a brick wall. What is the wall's basic building block? It is a single brick. Like a brick wall, cells are the building blocks that make up your body. Your body has many kinds of cells, each specialized for a specific purpose. Just as we use a variety of materials to build a home, the human body is constructed from many cell types. For example, epithelial cells protect the body's surface and cover the organs and body cavities within. Bone cells help to support and protect the body. Immune system cells fight invading bacteria. Additionally, blood and blood cells carry nutrients and oxygen throughout the body while removing carbon dioxide. Each of these cell types plays a vital role during the body's growth, development, and day-to-day maintenance. -

Atypical Centromeres in Plants—What They Can Tell Us Cuacos, Maria; H
University of Birmingham Atypical centromeres in plants—what they can tell us Cuacos, Maria; H. Franklin, F. Chris; Heckmann, Stefan DOI: 10.3389/fpls.2015.00913 License: Creative Commons: Attribution (CC BY) Document Version Publisher's PDF, also known as Version of record Citation for published version (Harvard): Cuacos, M, H. Franklin, FC & Heckmann, S 2015, 'Atypical centromeres in plants—what they can tell us', Frontiers in Plant Science, vol. 6, 913. https://doi.org/10.3389/fpls.2015.00913 Link to publication on Research at Birmingham portal Publisher Rights Statement: Eligibility for repository : checked 18/11/2015 General rights Unless a licence is specified above, all rights (including copyright and moral rights) in this document are retained by the authors and/or the copyright holders. The express permission of the copyright holder must be obtained for any use of this material other than for purposes permitted by law. •Users may freely distribute the URL that is used to identify this publication. •Users may download and/or print one copy of the publication from the University of Birmingham research portal for the purpose of private study or non-commercial research. •User may use extracts from the document in line with the concept of ‘fair dealing’ under the Copyright, Designs and Patents Act 1988 (?) •Users may not further distribute the material nor use it for the purposes of commercial gain. Where a licence is displayed above, please note the terms and conditions of the licence govern your use of this document. When citing, please reference the published version. Take down policy While the University of Birmingham exercises care and attention in making items available there are rare occasions when an item has been uploaded in error or has been deemed to be commercially or otherwise sensitive. -

Large-Scale Chromosomal Changes 1
Large-Scale Chromosomal Changes 1. MM N OO would be classified as 2n–1; MM NN OO would be classified as 2n; and MMM NN PP would be classified as 2n+1. 2. It would more likely be an autopolyploid. To make sure it was polyploid, you would need to microscopically examine stained chromosomes from mitotically dividing cells and count the chromosome number. 3. Aneuploid. Trisomic refers to three copies of one chromosome. Triploid refers to three copies of all chromosomes. 5. No. Amphidiploid means “doubled diploid.” Because cauliflower has n = 9 chromosome, it could not have arose in this fashion. It has, however, contributed to other amphidiploid species. 9. Cells destined to become pollen grains can be induced by cold treatment to grow into embryoids. These embryoids can then be grown on agar to form monoploid plantlets. 11. Yes. You would expect that one-sixth of the gametes would be a. Also, two-sixths would be A, two-sixths would be Aa, and one-sixth would be AA. 12. Both XXY and XXX would be fertile. XO (Turner) and XXY (Klinefelter) are both sterile. 13. Older mothers have an elevated risk of having a child with Down syndrome and other non-disjunctional events. 14. No. The DNA backbone has strict 5´ to 3´ polarity, and 5´ ends can only be joined to 3´ ends. 15. A crossover within a paracentric inversion heterozygote results in a dicentric bridge (and an acentric fragment). 16. An acentric fragment cannot be aligned or moved during meiosis (or mitosis) and consequently is lost. 20. -

The Mitochondrial Genome. the Nucleoid
ISSN 0006-2979, Biochemistry (Moscow), 2016, Vol. 81, No. 10, pp. 1057-1065. © Pleiades Publishing, Ltd., 2016. Original Russian Text © A. A. Kolesnikov, 2016, published in Biokhimiya, 2016, Vol. 81, No. 10, pp. 1322-1331. REVIEW The Mitochondrial Genome. The Nucleoid A. A. Kolesnikov Lomonosov Moscow State University, Faculty of Biology, 119991 Moscow, Russia; E-mail: [email protected] Received May 30, 2016 Revision received July 1, 2016 Abstract—Mitochondrial DNA (mtDNA) in cells is organized in nucleoids containing DNA and various proteins. This review discusses questions of organization and structural dynamics of nucleoids as well as their protein components. The structures of mt-nucleoid from different organisms are compared. The currently accepted model of nucleoid organization is described and questions needing answers for better understanding of the fine mechanisms of the mitochondrial genetic apparatus functioning are discussed. DOI: 10.1134/S0006297916100047 Key words: mitochondrial nucleoid, mitochondrial genome It is now clear that understanding of genome organ- mtDNA). In fact, proteins associated with a nucleoid can ization of subcellular structures is necessary for under- influence the speed of accumulation of mutations. standing of cellular processes occurring through the cell In the last decade, reviews devoted to this subject cycle, including questions of storage, realization, and appeared regularly [5-9]. Generally, they cover the subject transmission of genetic information. A considerable of nucleoid organization in metazoan mitochondria amount of information about forms and sizes of mito- (mainly in human cells) and fungi (mainly yeast). chondrial DNA (mtDNA) throughout evolution has been Reviews touching plant cells are much less frequent [10, accumulated [1]. -

The Force for Poleward Chromosome Motion in Haemanthus Cells Acts
The Force for Poleward Chromosome Motion in Haemanthus Cells Acts along the Length of the Chromosome during Metaphase but Only at the Kinetochore during Anaphase Alexey Khodjakov,* Richard W. Cole,* Andrew S. Bajer, ~ and Conly L. Rieder** *Laboratory of Cell Regulation, Wadsworth Center for Laboratories and Research, Albany, New York 12201-0509; ~Department of Biomedical Sciences, State University of New York, Albany, New York 12222; and§Department of Biology, University of Oregon, Eugene, Oregon 97403 Abstract. The force for poleward chromosome motion other force that now transported the fragments to the during mitosis is thought to act, in all higher organisms, spindle equator at 1.5-2.0 tzm/min. These fragments exclusively through the kinetochore. We have used then remained near the spindle midzone until phrag- time-lapse, video-enhanced, differential interference moplast development, at which time they were again contrast light microscopy to determine the behavior of transported randomly poleward but now at ~3/xm/min. kinetochore-free "acentric" chromosome fragments This behavior of acentric chromosome fragments on and "monocentric" chromosomes containing one kine- anastral plant spindles differs from that reported for tochore, created at various stages of mitosis in living the astral spindles of vertebrate cells, and demonstrates higher plant (Haemanthus) cells by laser microsurgery. that in forming plant spindles, a force for poleward Acentric fragments and monocentric chromosomes chromosome motion is generated independent of the generated during spindle formation and metaphase kinetochore. The data further suggest that the three both moved towards the closest spindle pole at a rate stages of non-kinetochore chromosome transport we (~1.0 txm/min) similar to the poleward motion of observed are all mediated by the spindle microtubules.