Avian Lampbrush Chromosomes: a Powerful Tool for Exploration of Genome Expression
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insights Into Hp1a-Chromatin Interactions
cells Review Insights into HP1a-Chromatin Interactions Silvia Meyer-Nava , Victor E. Nieto-Caballero, Mario Zurita and Viviana Valadez-Graham * Instituto de Biotecnología, Departamento de Genética del Desarrollo y Fisiología Molecular, Universidad Nacional Autónoma de México, Cuernavaca Morelos 62210, Mexico; [email protected] (S.M.-N.); [email protected] (V.E.N.-C.); [email protected] (M.Z.) * Correspondence: [email protected]; Tel.: +527773291631 Received: 26 June 2020; Accepted: 21 July 2020; Published: 9 August 2020 Abstract: Understanding the packaging of DNA into chromatin has become a crucial aspect in the study of gene regulatory mechanisms. Heterochromatin establishment and maintenance dynamics have emerged as some of the main features involved in genome stability, cellular development, and diseases. The most extensively studied heterochromatin protein is HP1a. This protein has two main domains, namely the chromoshadow and the chromodomain, separated by a hinge region. Over the years, several works have taken on the task of identifying HP1a partners using different strategies. In this review, we focus on describing these interactions and the possible complexes and subcomplexes associated with this critical protein. Characterization of these complexes will help us to clearly understand the implications of the interactions of HP1a in heterochromatin maintenance, heterochromatin dynamics, and heterochromatin’s direct relationship to gene regulation and chromatin organization. Keywords: heterochromatin; HP1a; genome stability 1. Introduction Chromatin is a complex of DNA and associated proteins in which the genetic material is packed in the interior of the nucleus of eukaryotic cells [1]. To organize this highly compact structure, two categories of proteins are needed: histones [2] and accessory proteins, such as chromatin regulators and histone-modifying proteins. -

Lecture No. VII Title of Topic: - Architecture of Chromosome Prepared By- Vinod Kumar, Assistant Professor, (PB & G) College of Agriculture, Powarkheda
Course: Fundamentals of Genetics Class: - Ist Year, IInd Semester Lecture No. VII Title of topic: - Architecture of Chromosome Prepared by- Vinod Kumar, Assistant Professor, (PB & G) College of Agriculture, Powarkheda Introduction:- E. Strasburger in 1875 first discovered thread-like structures which appeared during cell division. These thread like structures were called chromosomes due to their affinity for basic dyes. The term chromosome is derived from two Greek words; chrom = colour, soma=body. This term was first used by Waldeyer in 1888. Morphology: The outer covering or sheath of a chromosome is known as pellicle, which encloses the matrix. Within the matrix lies the chromatin. Flemming introduced the term chromatin in 1879. The term chromatin refers to the Feulgen positive materials observed in interphase nucleus and later during nuclear division. Chromatin readily stains with basic dyes especially Basic Fuchsin, which is specific for DNA which in turn is a major constituent of chromosomes. The chromosome morphology changes during cell division and mitotic metaphase is the most suitable stage for studies on chromosome morphology. In mitotic metaphase chromosomes, the following structural features can be seen under the light microscope. 1. Chromatid: Each metaphase chromosome appears to be longitudinally divided into two identical parts each of which is called chromatid. Both the chromatids of a chromosome appear to be joined together at a point known as centromere. The two chromatids of chromosome separate from each other during mitotic anaphase (and during anaphase II of meiosis) and move towards opposite poles. Since the two chromatids making up a chromosome are produced through replication of a single chromatid during synthesis (S) phase of interphase, they are referred to as sister chromatids. -

On the Positions of Centromeres in Chicken Lampbrush Chromosomes
Chromosome Research (2006) 14:777–789 # Springer 2006 DOI: 10.1007/s10577-006-1085-y On the positions of centromeres in chicken lampbrush chromosomes Alla Krasikova, Svetlana Deryusheva, Svetlana Galkina, Anna Kurganova, Andrei Evteev & Elena Gaginskaya* Biological Research Institute, Saint-Petersburg State University, Oranienbaumskoie sch. 2, Stary Peterhof, Saint-Petersburg, 198504, Russia; Tel: +7-812-4507311; Fax: +7-812-4507310; E-mail: [email protected] *Correspondence Received 5 May 2006. Received in revised form and accepted for publication by Mary Delany 24 July 2006 Key words: centromere, chicken, CNM, cohesin, lampbrush chromosome Abstract Using immunostaining with antibodies against cohesin subunits, we show here that cohesin-enriched structures analogous to the so-called centromere protein bodies (PB) are the characteristic of galliform lampbrush chromosomes. Their centromeric location was verified by FISH with certain DNA probes. PB-like structures were used as markers for centromere localization in chicken lampbrush chromosomes. The gap predicted to be centromeric in current chicken chromosome 3 sequence assembly was found to correspond to the non- centromeric cluster of CNM repeat on the q-arm of chromosome 3; the centromere is proposed to be placed at another position. The majority of chicken microchromosomes were found to be acrocentric, in contrast to Japanese quail microchromosomes which are biarmed. Centromere cohesin-enriched structures on chicken and quail lampbrush microchromosomes co-localize with pericentromeric CNM and BglIIj repeats respectively. FISH to the nascent transcripts on chicken lampbrush chromosomes revealed numerous non-centromeric CNM clusters in addition to pericentromeric arrays. Complementary CNM transcripts from both C- and G-rich DNA strands were revealed during the lampbrush stage. -

Mapping Epigenetic Modifications on Chicken Lampbrush Chromosomes Tatiana Kulikova , Anna Surkova , Anna Zlotina and Alla Krasikova*
Kulikova et al. Molecular Cytogenetics (2020) 13:32 https://doi.org/10.1186/s13039-020-00496-0 RESEARCH Open Access Mapping epigenetic modifications on chicken lampbrush chromosomes Tatiana Kulikova , Anna Surkova , Anna Zlotina and Alla Krasikova* Abstract Background: The epigenetic regulation of genome is crucial for implementation of the genetic program of ontogenesis through establishing and maintaining differential gene expression. Thus mapping of various epigenetic modifications to the genome is relevant for studying the regulation of gene expression. Giant transcriptionally active lampbrush chromosomes are an established tool for high resolution physical mapping of the genome and its epigenetic modifications. This study is aimed at characterizing the epigenetic status of compact chromatin domains (chromomeres) of chicken lampbrush macrochromosomes. Results: Distribution of three epigenetic modifications – 5-methylcytosine, histone H3 trimethylated at lysine 9 and hyperacetylated histone H4 – along the axes of chicken lampbrush chromosomes 1–4, Z and W was analyzed in details. Enrichment of chromatin domains with the investigated epigenetic modifications was indicated on the cytological chromomere-loop maps for corresponding chicken lampbrush chromosomes. Heterogeneity in the distribution of 5-methylcytosine and histone H3 trimethylated at lysine 9 along the chromosome axes was revealed. Conclusions: On examples of certain chromomeres of chicken lampbrush chromosomes 1, 3, 4 and W we demonstrated that a combination of immunofluorescent staining and fluorescence in situ hybridization allows to relate the epigenetic status and a DNA sequence context of individual chromomeres. Keywords: Chromatin domain, Chromomere, Cytological chromomere-loop map, Chicken, Epigenetic modifications, Gene mapping, Histone modifications, Karyotype, Lampbrush chromosomes, Methylated cytosine, Tandem repeats Background active chromatin is organized in paired lateral loops out- Lampbrush chromosomes are highly extended transcrip- going from the chromomeres. -
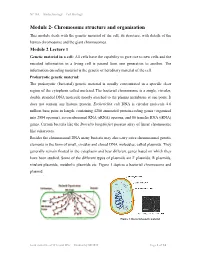
Chromosome Structure and Organisation
NPTEL – Biotechnology – Cell Biology Module 2- Chromosome structure and organisation This module deals with the genetic material of the cell, its structure, with details of the human chromosome and the giant chromosomes. Module 2 Lecture 1 Genetic material in a cell: All cells have the capability to give rise to new cells and the encoded information in a living cell is passed from one generation to another. The information encoding material is the genetic or hereditary material of the cell. Prokaryotic genetic material: The prokaryotic (bacterial) genetic material is usually concentrated in a specific clear region of the cytoplasm called nucleiod. The bacterial chromosome is a single, circular, double stranded DNA molecule mostly attached to the plasma membrane at one point. It does not contain any histone protein. Escherichia coli DNA is circular molecule 4.6 million base pairs in length, containing 4288 annotated protein-coding genes (organized into 2584 operons), seven ribosomal RNA (rRNA) operons, and 86 transfer RNA (tRNA) genes. Certain bacteria like the Borrelia burgdorferi possess array of linear chromosome like eukaryotes. Besides the chromosomal DNA many bacteria may also carry extra chromosomal genetic elements in the form of small, circular and closed DNA molecules, called plasmids. They generally remain floated in the cytoplasm and bear different genes based on which they have been studied. Some of the different types of plasmids are F plasmids, R plasmids, virulent plasmids, metabolic plasmids etc. Figure 1 depicts a bacterial chromosome and plasmid. Figure 1: Bacterial genetic material Joint initiative of IITs and IISc – Funded by MHRD Page 1 of 24 NPTEL – Biotechnology – Cell Biology Virus genetic material: The chromosomal material of viruses is DNA or RNA which adopts different structures. -

Lampbrush Chromosomes
COMMENTARY Lampbrush chromosomes H. C. MACGREGOR Department of'/.oology, Univeisity of Leicester, Leicester LEI 7RH, UK 1986 was a celebration year for lampbrush chromo- problems of the fate and significance of the massive somes (LBCs), marked by the publication of Callan's amounts of non-coding transcripts, and the manner in comprehensive and authoritative book on these struc- which certain initiation sites are 'selected' for high-level tures. My commentary begins where Callan's book transcription on lateral loops, whereas others remain ends, standing on tiptoe beside a large and rather relatively inactive on or within the chromomeres. Of neat assembly of well-established facts, principles and particular interest are the recent studies of Epstein et hypotheses, and trying hard to catch a glimpse of what al. (1986) and Epstein & Gall (1987). These investi- lies ahead. gators have focussed their attention on the nature and Several major principles can now be accepted with transcription of the 330 base-pair (bp) satellite DNA of confidence. LBCs are a feature of the growing oocytes Notophthalmus viridescens (Nv2). Nv2 occurs in tan- of most animals, except mammals and certain insects. demly repeated clusters throughout the genome and its Callan (1986) has reviewed this principle in depth, and sequence is highly conserved among the salamandrid has discussed the significance of exceptional cases. The species so far examined (Epstein et al. 1986). It is main defining feature of LBCs, their lateral loops, are transcribed on lampbrush loops by read-through from regions of intense RNA synthesis, and together these adjacent structural gene promoters, and homologous loops produce a large variety of transcription products, strand-specific cytoplasmic transcripts are found in the though not all coding sequences in the genome are cytoplasm of a variety of tissues. -

Comparison of Active Transcription Regions of Lampbrush Chromosomes with the Mitotic Chromosome G Pattern in the European Domestic Goose Anser Anser
Archiv Tierzucht 54 (2011) 1, 69-82, ISSN 0003-9438 69 © Leibniz Institute for Farm Animal Biology, Dummerstorf, Germany Comparison of active transcription regions of lampbrush chromosomes with the mitotic chromosome G pattern in the European domestic goose Anser anser Katarzyna Andraszek and Elżbieta Smalec Department of Animal Genetics and Horse Breeding, Institute of Bioengineering and Animal Breeding, University of Life Sciences and Humanities, Poland Abstract The most complete information on the karyotype is acquired through the observation of chromosomes obtained from dividing cells. A high number of chromosomes and the presence of microchromosomes in the bird karyotype have made cytogeneticists look for other sources of information on chromosomes. Information sources of great value for the bird karyotype analysis are meiotic chromosomes, specifically represented by lampbrush chromosomes. Lampbrush chromosomes (LBCs) found in developing oocytes of birds are perceived as a new model in cytogenetics which is especially important in the analysis of bird chromosomes. A typical LBC analysis enables one to assess transcription activity on the basis of LBC morphology (inactive chromomeres and side loops). A comparison of lampbrush chromosome transcription activity and the GTG pattern of the corresponding mitotic chromosomes have proven that active transcription regions with side loops correspond to G-positive bands on mitotic chromosomes. Keywords: geese, Anser anser, lampbrush chromosomes (LBCs), mitotic chromosomes, transcription, G-bands Zusammenfassung Vergleich zwischen den transkriptionell aktiven Regionen der Lampenbürstenchromosomen und dem G-Muster der mitotischen Chromosomen der Europäischen Hausgans Anser anser Die vollständigsten Informationen über den Karyotyp erhält man durch die Beobachtung von Chromosomen sich teilendender Zellen. Die große Anzahl von Chromosomen und das Vorhandensein von Mikrochromosomen im Vogelkaryotyp haben Zytogenetiker zum Suchen von anderen Informationsquellen über Chromosomen veranlasst. -

1 Are Lampbrush Chromosomes Unique to Meiotic Cells? Joseph G
1 Are lampbrush chromosomes unique to meiotic cells? Joseph G. Gall Department of Embryology, Carnegie Institution for Science, Baltimore MD 21218 Corresponding author: Joseph G. Gall Department of Embryology Carnegie Institution for Science 3520 San Martin Drive Baltimore, MD 21218 Phone: (410) 246-3017 e-mail: [email protected] Running title: Lampbrush chromosomes and meiosis Key words: lampbrush chromosome, meiosis, oocyte, oogenesis, polyploidy, reprogramming, transcription. Abbreviations: GV, germinal vesicle; LBC, lampbrush chromosome; pol II, polymerase II; TU, transcription unit. 2 Abstract Lampbrush chromosomes (LBCs) are transcriptionally active chromosomes found in the germinal vesicle (GV) of large oocytes of many vertebrate and invertebrate animals and also in the giant single-celled alga Acetabularia. These cells are all in prophase of the first meiotic division. Nevertheless, many meiotic cells do not develop LBCs, arguing that LBCs are not an essential feature of meiosis. LBCs probably represent the most active transcriptional state that can be attained by cells that must give rise to diploid progeny. Polyploidy permits cells to reach higher rates of transcription per nucleus but precludes a return to diploidy. In this sense LBCs represent a relatively inefficient transcriptional compromise employed by large meiotic cells. These considerations help to explain why transcriptionally active GVs develop LBCs, but they do not explain why LBCs have never been seen in somatic cells, diploid or otherwise. If LBCs are truly limited to germ cells, then some of their unusual features may reflect reprogramming of the genome. If this is the case, LBCs provide unique opportunities to study reprogramming at the level of the individual transcription unit. -

Visualization of Early Chromosome Condensation: a Hierarchical Folding, Axial Glue Model of Chromosome Structure
JCBArticle Visualization of early chromosome condensation: a hierarchical folding, axial glue model of chromosome structure Natashe Kireeva,1 Margot Lakonishok,1 Igor Kireev,1 Tatsuya Hirano,2 and Andrew S. Belmont1 1Department of Cell and Structural Biology, University of Illinois at Urbana-Champaign, Urbana, IL 61801 2Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724 urrent models of mitotic chromosome structure are appear until late prophase, after formation of uniformly based largely on the examination of maximally con- condensed middle prophase chromosomes. Instead, SMC2 C densed metaphase chromosomes. Here, we test these associates throughout early and middle prophase chroma- models by correlating the distribution of two scaffold com- tids, frequently forming foci over the chromosome exterior. ponents with the appearance of prophase chromosome Early prophase condensation occurs through folding of folding intermediates. We confirm an axial distribution of large-scale chromatin fibers into condensed masses. These topoisomerase II␣ and the condensin subunit, structural resolve into linear, 200–300-nm-diameter middle prophase maintenance of chromosomes 2 (SMC2), in unextracted chromatids that double in diameter by late prophase. We metaphase chromosomes, with SMC2 localizing to a 150– propose a unified model of chromosome structure in which 200-nm-diameter central core. In contrast to predictions of hierarchical levels of chromatin folding are stabilized late radial loop/scaffold models, this axial distribution does not in mitosis by an axial “glue.” Introduction Understanding the structural and molecular basis of mitotic predictions in terms of the functional mechanisms under- chromosome condensation remains a basic challenge in cell lying chromosome condensation and the types of folding biology. -

Structure of Lampbrush Chromosome Loops During Different States of Transcriptional Activity As Visualized in the Presence
Biology of the Cell, 59 (1987) 33-42 33 © Elsevier, Paris Original article Structure of lampbrush chromosome loops during different states of transcriptional activity as visualized in the presence I of physiological salt concentrations l,. Ulrich SCHEER * Division of Membrane Biology and Biochemistry, Institute of Cell and Tumor Biology, German Cancer Research Center, D-6900 Heidelberg, F.R.G. (Received 11-7-1986; accepted 17-11-1986) Lampbrush chromosomes of amphibian oocytes were isolated in the presence of near-physiological salt concentrations, to preserve their native state, and studied by electron microscopy of ultrathin s~dions. The transcriptional state of the lampbrush chromosomes was experimentally modulated by incubating the oocytes for various time periods in medium containing actinomycin D. The observations show that the structure of the lateral loops changes rapidly in response to alterations in transcriptional activity. During decreasing transcriptional activity and reduced packing density of transcripts, the chromatin axis first condensed into nucleosomes and then into an approximately 30 nm thick higher order chromatin fiber. Packaging of the loop axis into supranucleosomal structures may contribute to the foreshortening and retraction of the loops observed during inhibition of transcription and in later stages of meiotic prophase. The increasing packing density of the DNA during the retraction process of the loops could also be visualized by immunofluorescence microscopy using antibodies to DNA. The dependence of the loop chromatin structure on transcriptional activity is discussed in relation to current views of mechanisms involved in gene activation. lampbrush chromosomes - chromatin structure - electron microscopy - immunofluorescence microscopy - DNA antibodies INTRODUCTION transcribing RN A polymerases the chromatin fiber is largely extended and nucleosomes are absent [13]. -

A Method for Determination of the in Situ Distribution of Chromosomal Proteins
Proc. Nat. Acad. Sci. USA Vol. 73, No. 2, pp. 423-427, February 1976 Biochemistry A method for determination of the in situ distribution of chromosomal proteins (nonhistone chromosomal proteins/chromosome structure/Drosophila melanogaster/polytene chromosomes/immunofluorescence) LEE M. SILVER* AND SARAH C. R. ELGINt Committee on Higher Degrees in Biophysics and t Department of Biochemistry and Molecular Biology, The Biological Laboratories, Harvard University, Cambridge, Massac usetts 02138 Communicated by Matthew S. Meselson, December 1, 1975 ABSTRACT A technique has been developed for "stain- tracted from chromatin with 1.6 M NaCl-0.2 M HC1. The ing" cytological preparations by indirect immunofluorescent DNA and NHC proteins were solubilized in 0.05 M Tris, pH methods that permits determination of the in situ distribu- tion of chromosomal proteins. The method is particularly ori- 8-1% sodium dodecyl sulfate (NaDodSO4), and the DNA ented to the use of polytene chromosome squashes from Dro- was removed by centrifugation (3). The supernatant con- sophila salivary glands. Control experiments indicate that tains the NHC proteins; this preparation will be referred to the fixation methods used allow little or no extraction or re- as NHCP-1. (ii) Chromatin was dissociated in 5 M urea-2 M arrangement of the chromosomal proteins. The results ob- NaCl-0.001 M sodium phosphate buffer, pH 7.0-1 mM so- tained demonstrate the specific in vivo chromosomal loca- tions of nonhistone proteins purified from isolated chroma- dium bisulfite. The histones, NHC proteins, and DNA were tin. The technique is apparently capable of resolution at the then eluted sequentially from a hydroxylapatite column level of the chromomere or band, the unit of genetic organi- with 0.001, 0.1, and 0.5 M phosphate (4). -

Chromomeres Revisited
Chromosome Res DOI 10.1007/s10577-012-9310-3 Chromomeres revisited Herbert C. Macgregor # Springer Science+Business Media B.V. 2012 Abstract The history of studies on the chromomeres Keywords lampbrush chromosome . chromomeres . of lampbrush chromosomes is outlined and evidence chromatin . transcription for the nature and function of these structures is col- lected and summarised. Chromomeres and their asso- Abbreviations ciated loops on lampbrush chromosomes are not bp Base pairs genetic units although in some special cases, they CENP-A Centromeric protein A consist of specific families of repeated DNA sequen- DAPI 4′,6-Diamidino-2-phenylindole ces. The emergence of a chromomeric organisation LBC Lampbrush chromosome coincides with the onset and intensification of tran- H4 Histone H4 scription on lampbrush loops. Modern molecular stud- H3K4me3 A modified histone H3 that marks ies have provided evidence that the chromatin of transcriptional activation lampbrush chromomeres differs in several important HMGN High molility group nucleosome binding respects from that of condensed metaphase chromo- proteins somes. It is in a highly dynamic state that facilitates HP A protein involved in heterochromatin localised transcription whilst keeping the chromosome formation and gene silencing safe from structural changes that might impede its SMC Structural maintenance of chromosome orderly progression up to and through meiotic meta- protein phase 1. Lampbrush chromosomes (LBCs) are a phys- RAD21 A protein involved in chromosome ically induced phenomenon, facilitated by the condensation in mitosis selective absence of molecular factors that would XCAP-D2 A condensin complex with a role in interfere with their main transcriptional role. LBC chromosome condensation and segregation morphology is highly dynamic and driven by tran- scriptive activity.