A Glimpse of Plants in Medog
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Living in Drylands: Functional Adaptations of Trees and Shrubs to Cope with High Temperatures and Water Scarcity
Review Living in Drylands: Functional Adaptations of Trees and Shrubs to Cope with High Temperatures and Water Scarcity José Javier Peguero-Pina 1,2,* , Alberto Vilagrosa 3 , David Alonso-Forn 1 , Juan Pedro Ferrio 1,4 , Domingo Sancho-Knapik 1,2 and Eustaquio Gil-Pelegrín 1 1 Unidad de Recursos Forestales, Centro de Investigación y Tecnología Agroalimentaria de Aragón (CITA), Avda. Montañana 930, 50059 Zaragoza, Spain; [email protected] (D.A.-F.); [email protected] (J.P.F.); [email protected] (D.S.-K.); [email protected] (E.G.-P.) 2 Instituto Agroalimentario de Aragón -IA2- (CITA-Universidad de Zaragoza), 50059 Zaragoza, Spain 3 Mediterranean Center for Environmental Studies (Fundación CEAM), Joint Research Unit University of Alicante—CEAM, Univ. Alicante, PO Box 99, 03080 Alicante, Spain; [email protected] 4 Aragon Agency for Research and Development (ARAID), E-50018 Zaragoza, Spain * Correspondence: [email protected]; Tel.: +34-976-716-974 Received: 5 August 2020; Accepted: 22 September 2020; Published: 23 September 2020 Abstract: Plant functioning and survival in drylands are affected by the combination of high solar radiation, high temperatures, low relative humidity, and the scarcity of available water. Many ecophysiological studies have dealt with the adaptation of plants to cope with these stresses in hot deserts, which are the territories that have better evoked the idea of a dryland. Nevertheless, drylands can also be found in some other areas of the Earth that are under the Mediterranean-type climates, which imposes a strong aridity during summer. In this review, plant species from hot deserts and Mediterranean-type climates serve as examples for describing and analyzing the different responses of trees and shrubs to aridity in drylands, with special emphasis on the structural and functional adaptations of plants to avoid the negative effects of high temperatures under drought conditions. -

Conservation Assessment for the Bigleaf Snowbell (Styrax Grandifolius Ait.)
Conservation Assessment for the Bigleaf Snowbell (Styrax grandifolius Ait.) Steven R. Hill, Ph.D. Division of Biodiversity and Ecological Entomology Biotic Surveys and Monitoring Section 1816 South Oak Street Champaign, Illinois 61820 Prepared for the U.S.D.A. Forest Service, Eastern Region (Region 9), Shawnee and Hoosier National Forests INHS Technical Report 2007 (65) Date of Issue: 17 December 2007 Cover photo: Styrax grandifolius Ait., from the website: In Bloom – A Monthly Record of Plants in Alabama; Landscape Horticulture at Auburn University, Auburn, Alabama. http://www.ag.auburn.edu/hort/landscape/inbloomapril99.html This Conservation Assessment was prepared to compile the published and unpublished information on the subject taxon or community; or this document was prepared by another organization and provides information to serve as a Conservation Assessment for the Eastern Region of the Forest Service. It does not represent a management decision by the U.S. Forest Service. Though the best scientific information available was used and subject experts were consulted in preparation of this document, it is expected that new information will arise. In the spirit of continuous learning and adaptive management, if you have information that will assist in conserving the subject taxon, please contact the Eastern Region of the Forest Service - Threatened and Endangered Species Program at 310 Wisconsin Avenue, Suite 580 Milwaukee, Wisconsin 53203. 2 Conservation Assessment for the Bigleaf Snowbell (Styrax grandifolius Ait.) Table of Contents -

Illicium Parviflorum1
Fact Sheet FPS-278 October, 1999 Illicium parviflorum1 Edward F. Gilman2 Introduction This rapidly growing, large, evergreen, Florida native shrub has medium- to coarse-textured, olive green, leathery leaves and small, greenish-yellow flowers (Fig. 1). The many slender, drooping branches of Anise give a rounded, open canopy in the shade, ideal for natural settings, or can be pruned into dense hedges, screens, or windbreaks in sunny locations. Branches often root when they touch the ground and root sprouts appear several years after planting. This adds to the density of the shrub. The slightly fragrant spring flowers are followed by brown, star-shaped, many-seeded pods which cling to the stems. The leaves of Anise give off a distinctive fragrance of licorice when bruised or crushed. General Information Scientific name: Illicium parviflorum Pronunciation: ill-LISS-see-um par-vif-FLOR-um Common name(s): Anise Family: Illiciaceae Plant type: tree Figure 1. Anise. USDA hardiness zones: 7B through 10A (Fig. 2) Planting month for zone 7: year round Planting month for zone 8: year round Planting month for zone 9: year round Description Planting month for zone 10: year round Height: 15 to 20 feet Origin: native to Florida Spread: 10 to 15 feet Uses: hedge; espalier; screen; foundation; border Plant habit: oval Availablity: generally available in many areas within its Plant density: dense hardiness range Growth rate: moderate Texture: medium 1.This document is Fact Sheet FPS-278, one of a series of the Environmental Horticulture Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. -

Garden Mastery Tips March 2006 from Clark County Master Gardeners
Garden Mastery Tips March 2006 from Clark County Master Gardeners Flowering Quince Flowering quince is a group of three hardy, deciduous shrubs: Chaenomeles cathayensis, Chaenomeles japonica, and Chaenomeles speciosa. Native to eastern Asia, flowering quince is related to the orchard quince (Cydonia oblonga), which is grown for its edible fruit, and the Chinese quince (Pseudocydonia sinensis). Flowering quince is often referred to as Japanese quince (this name correctly refers only to C. japonica). Japonica is often used regardless of species, and flowering quince is still called Japonica by gardeners all over the world. The most commonly cultivated are the hybrid C. superba and C. speciosa, not C. japonica. Popular cultivars include ‘Texas Scarlet,’ a 3-foot-tall plant with red blooms; ‘Cameo,’ a double, pinkish shrub to five feet tall; and ‘Jet Trail,’ a white shrub to 3 feet tall. Flowering quince is hardy to USDA Zone 4 and is a popular ornamental shrub in both Europe and North America. It is grown primarily for its bright flowers, which may be red, pink, orange, or white. The flowers are 1 to 2 inches in diameter, with five petals, and bloom in late winter or early spring. The glossy dark green leaves appear soon after flowering and turn yellow or red in autumn. The edible quince fruit is yellowish-green with reddish blush and speckled with small dots. The fruit is 2 to 4 inches in diameter, fragrant, and ripens in fall. The Good The beautiful blossoms of flowering quince Flowering quince is an easy-to-grow, drought-tolerant shrub that does well in shady spots as well as sun (although more sunlight will produce better flowers). -

Chaenomeles Speciosa) in the Naxi and Tibetan Highlands of NW Yunnan, China
Cultural and Ecosystem Services of Flowering Quince (Chaenomeles speciosa) in the Naxi and Tibetan Highlands of NW Yunnan, China. Authors: Lixin Yang, Selena Ahmed, John Richard Stepp, Yanqinag Zhao, Ma Jun Zeng, Shengji Pei, Dayuan Xue, and Gang Xu The final publication is available at Springer via https://dx.doi.org/10.1007/s12231-015-9318-7. Yang, Lixin, Selena Ahmed, John Richard Stepp, Yanqinag Zhao, Ma Jun Zeng, Shengji Pei, Dayuan Xue, and Gang Xu. “Cultural Uses, Ecosystem Services, and Nutrient Profile of Flowering Quince (Chaenomeles Speciosa) in the Highlands of Western Yunnan, China.” Economic Botany 69, no. 3 (September 2015): 273–283. doi:10.1007/s12231-015-9318-7. Made available through Montana State University’s ScholarWorks scholarworks.montana.edu Cultural Uses, Ecosystem Services, and Nutrient Profile Chaenomeles speciosa of Flowering Quince ( ) in the Highlands 1 of Western Yunnan, China 2,3 3,4 ,3,5 6 LIXIN YANG ,SELENA AHMED ,JOHN RICHARD STEPP* ,YANQINAG ZHAO , 7 2 ,3 2 MA JUN ZENG ,SHENGJI PEI ,DAYUAN XUE* , AND GANG XU 2State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institutes of Botany, Chinese Academy of Sciences, Kunming, China 3College of Life and Environmental Science, Minzu University of China, Beijing, China 4Department of Health and Human Development, Montana State University, Bozeman, MT, USA 5Department of Anthropology, University of Florida, Gainesville, FL, USA 6College of Forestry and Vocational Technology in Yunnan, Kunming, China 7Southwest Forestry University, Bailongshi, Kunming, China *Corresponding author; e-mail: [email protected]; [email protected] Introduction ample light but is tolerant of partial shade. -

Illicium Floridanum1
Fact Sheet FPS-277 October, 1999 Illicium floridanum1 Edward F. Gilman2 Introduction This rapidly growing, evergreen, Florida native shrub has olive green leaves and reddish-purple, starry, two-inch flowers (Fig. 1). The many slender branches of Florida Anise droop to the ground giving a rounded, open canopy in the shade, ideal for natural settings, or in sunny locations it can be pruned into dense hedges or windbreaks. The small, somewhat showy, maroon flowers appear in spring and are followed in late summer to fall by star-shaped, many-seeded pods which cling to the stems. The leaves of Florida Anise give off a distinctive odor when bruised or crushed. General Information Scientific name: Illicium floridanum Pronunciation: ill-LISS-see-um flor-rid-DAY-num Common name(s): Florida Anise-Tree, Florida Anise Family: Illiciaceae Plant type: shrub USDA hardiness zones: 8 through 10 (Fig. 2) Planting month for zone 7: year round Figure 1. Florida Anise-Tree. Planting month for zone 8: year round Planting month for zone 9: year round Planting month for zone 10: year round Description Origin: native to Florida Height: 10 to 15 feet Uses: container or above-ground planter; hedge; espalier; Spread: 6 to 10 feet screen; foundation; border Plant habit: oval Availablity: somewhat available, may have to go out of the Plant density: dense region to find the plant Growth rate: moderate Texture: medium 1.This document is Fact Sheet FPS-277, one of a series of the Environmental Horticulture Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. -

Evaluation of Anti-Inflammatory Action of Illicium Verum - an in Vitro Study Rachel Paul1, R
Research Article Evaluation of anti-inflammatory action of Illicium verum - An in vitro study Rachel Paul1, R. V. Geetha2* ABSTRACT Introduction: Illicium verum is a medium-sized evergreen tree native to northeast Vietnam and southwest China. A spice commonly called star anise. Star anise refers to aromatic herbs which are used in cooking for their distinctive flavor and their fragrance. Star anise is the major source of the chemical compound, shikimic acid which is a pharmaceutical synthesis of anti- influenza drug. It also has raw materials needed for fermentation of the food. Star anise has anti-inflammatory, antimicrobial, antifungal, and antioxidant properties. It has many medicinal properties which can also be used to treat cancer as well as gastric problems. It is an easily available herb in the market and is easily affordable by many people; it can be used in the treatment of various diseases. Materials and Methods: The anti-inflammatory activity was studied using protein denaturation assay and the results were read spectrophotometrically. Results: The anti-inflammatory activity of the extract was studied by its ability to inhibit protein denaturation. It was effective in inhibiting heat induced albumin denaturation at different concentrations. Maximum inhibition, 77.87 ± 1.55 was observed at 500 µg/ml. Half-maximal inhibitory concentration value was found to be 105.35 ± 1.99 µg/ml. Conclusion: The result obtained was compared to the commonly available nonsteroidal anti- inflammatory drugs such as aspirin. This research conducted -

Tree of the Year: Liquidambar Eric Hsu and Susyn Andrews
Tree of the Year: Liquidambar Eric Hsu and Susyn Andrews With contributions from Anne Boscawen (UK), John Bulmer (UK), Koen Camelbeke (Belgium), John Gammon (UK), Hugh Glen (South Africa), Philippe de Spoelberch (Belgium), Dick van Hoey Smith (The Netherlands), Robert Vernon (UK) and Ulrich Würth (Germany). Affinities, generic distribution and fossil record Liquidambar L. has close taxonomic affinities with Altingia Noronha since these two genera share gum ducts associated with vascular bundles, terminal buds enclosed within numerous bud scales, spirally arranged stipulate leaves, poly- porate (with several pore-like apertures) pollen grains, condensed bisexual inflorescences, perfect or imperfect flowers, and winged seeds. Not surpris- ingly, Liquidambar, Altingia and Semiliquidambar H.T. Chang have now been placed in the Altingiaceae, as originally treated (Blume 1828, Wilson 1905, Chang 1964, Melikan 1973, Li et al. 1988, Zhou & Jiang 1990, Wang 1992, Qui et al. 1998, APG 1998, Judd et al. 1999, Shi et al. 2001 and V. Savolainen pers. comm.). These three genera were placed in the subfamily Altingioideae in Hamamelidaceae (Reinsch 1890, Chang 1979, Cronquist 1981, Bogle 1986, Endress 1989) or the Liquidambaroideae (Harms 1930). Shi et al. (2001) noted that Altingia species are evergreen with entire, unlobed leaves; Liquidambar is deciduous with 3-5 or 7-lobed leaves; while Semiliquidambar is evergreen or deciduous, with trilobed, simple or one-lobed leaves. Cytological studies have indicated that the chromosome number of Liquidambar is 2n = 30, 32 (Anderson & Sax 1935, Pizzolongo 1958, Santamour 1972, Goldblatt & Endress 1977). Ferguson (1989) stated that this chromosome number distinguished Liquidambar from the rest of the Hamamelidaceae with their chromosome numbers of 2n = 16, 24, 36, 48, 64 and 72. -

Styrax Japonicus Japanese Snowbell1 Edward F
Fact Sheet ST-605 October 1994 Styrax japonicus Japanese Snowbell1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Japanese Snowbell is a small deciduous tree that slowly grows from 20 to 30 feet in height and has rounded canopy with a horizontal branching pattern (Fig. 1). With lower branches removed, it forms a more vase-shaped patio-sized shade tree. The smooth, attractive bark has orange-brown interlacing fissures adding winter interest to any landscape. The white, bell-shaped, drooping flower clusters of Japanese Snowbell are quite showy in May to June. GENERAL INFORMATION Scientific name: Styrax japonicus Pronunciation: STY-racks juh-PAWN-ih-kuss Common name(s): Japanese Snowbell Figure 1. Middle-aged Japanese Snowbell. Family: Styracaceae USDA hardiness zones: 6 through 8A (Fig. 2) DESCRIPTION Origin: not native to North America Uses: container or above-ground planter; large Height: 20 to 30 feet parking lot islands (> 200 square feet in size); wide Spread: 15 to 25 feet tree lawns (>6 feet wide); medium-sized parking lot Crown uniformity: symmetrical canopy with a islands (100-200 square feet in size); medium-sized regular (or smooth) outline, and individuals have more tree lawns (4-6 feet wide); recommended for buffer or less identical crown forms strips around parking lots or for median strip plantings Crown shape: round; vase shape in the highway; near a deck or patio; trainable as a Crown density: moderate standard; small parking lot islands (< 100 square feet Growth rate: slow in size); narrow tree lawns (3-4 feet wide); specimen; Texture: medium sidewalk cutout (tree pit); residential street tree; no proven urban tolerance Availability: grown in small quantities by a small number of nurseries 1. -
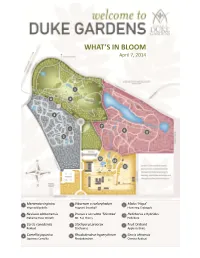
What's in Bloom
WHAT’S IN BLOOM April 7, 2014 5 4 6 2 7 1 9 8 3 12 10 11 1 Mertensia virginica 5 Viburnum x carlcephalum 9 Malus ‘Hopa’ Virginia Bluebells Fragrant Snowball Flowering Crabapple 2 Neviusia alabamensis 6 Prunus x serrulata ‘Shirotae’ 10 Helleborus x hybridus Alabama Snow Wreath Mt. Fuji Cherry Hellebore 3 Cercis canadensis 7 Stachyurus praecox 11 Fruit Orchard Redbud Stachyurus Apple cultivars 4 Camellia japonica 8 Rhododendron hyperythrum 12 Cercis chinensis Japanese Camellia Rhododendron Chinese Redbud WHAT’S IN BLOOM April 7, 2014 BLOMQUIST GARDEN OF NATIVE PLANTS Amelanchier arborea Common Serviceberry Sanguinaria canadensis Bloodroot Cornus florida Flowering Dogwood Stylophorum diphyllum Celandine Poppy Thalictrum thalictroides Rue Anemone Fothergilla major Fothergilla Trillium decipiens Chattahoochee River Trillium Hepatica nobilis Hepatica Trillium grandiflorum White Trillium Hexastylis virginica Wild Ginger Hexastylis minor Wild Ginger Trillium pusillum Dwarf Wakerobin Illicium floridanum Florida Anise Tree Trillium stamineum Blue Ridge Wakerobin Malus coronaria Sweet Crabapple Uvularia sessilifolia Sessileleaf Bellwort Mertensia virginica Virginia Bluebells Pachysandra procumbens Allegheny spurge Prunus americana American Plum DORIS DUKE CENTER GARDENS Camellia japonica Japanese Camellia Pulmonaria ‘Diana Clare’ Lungwort Cercis canadensis Redbud Prunus persica Flowering Peach Puschkinia scilloides Striped Squill Cercis chinensis Redbud Sanguinaria canadensis Bloodroot Clematis armandii Evergreen Clematis Spiraea prunifolia Bridalwreath -

Ecology and Ex Situ Conservation of Vanilla Siamensis (Rolfe Ex Downie) in Thailand
Kent Academic Repository Full text document (pdf) Citation for published version Chaipanich, Vinan Vince (2020) Ecology and Ex Situ Conservation of Vanilla siamensis (Rolfe ex Downie) in Thailand. Doctor of Philosophy (PhD) thesis, University of Kent,. DOI Link to record in KAR https://kar.kent.ac.uk/85312/ Document Version UNSPECIFIED Copyright & reuse Content in the Kent Academic Repository is made available for research purposes. Unless otherwise stated all content is protected by copyright and in the absence of an open licence (eg Creative Commons), permissions for further reuse of content should be sought from the publisher, author or other copyright holder. Versions of research The version in the Kent Academic Repository may differ from the final published version. Users are advised to check http://kar.kent.ac.uk for the status of the paper. Users should always cite the published version of record. Enquiries For any further enquiries regarding the licence status of this document, please contact: [email protected] If you believe this document infringes copyright then please contact the KAR admin team with the take-down information provided at http://kar.kent.ac.uk/contact.html Ecology and Ex Situ Conservation of Vanilla siamensis (Rolfe ex Downie) in Thailand By Vinan Vince Chaipanich November 2020 A thesis submitted to the University of Kent in the School of Anthropology and Conservation, Faculty of Social Sciences for the degree of Doctor of Philosophy Abstract A loss of habitat and climate change raises concerns about change in biodiversity, in particular the sensitive species such as narrowly endemic species. Vanilla siamensis is one such endemic species. -

Orchid Historical Biogeography, Diversification, Antarctica and The
Journal of Biogeography (J. Biogeogr.) (2016) ORIGINAL Orchid historical biogeography, ARTICLE diversification, Antarctica and the paradox of orchid dispersal Thomas J. Givnish1*, Daniel Spalink1, Mercedes Ames1, Stephanie P. Lyon1, Steven J. Hunter1, Alejandro Zuluaga1,2, Alfonso Doucette1, Giovanny Giraldo Caro1, James McDaniel1, Mark A. Clements3, Mary T. K. Arroyo4, Lorena Endara5, Ricardo Kriebel1, Norris H. Williams5 and Kenneth M. Cameron1 1Department of Botany, University of ABSTRACT Wisconsin-Madison, Madison, WI 53706, Aim Orchidaceae is the most species-rich angiosperm family and has one of USA, 2Departamento de Biologıa, the broadest distributions. Until now, the lack of a well-resolved phylogeny has Universidad del Valle, Cali, Colombia, 3Centre for Australian National Biodiversity prevented analyses of orchid historical biogeography. In this study, we use such Research, Canberra, ACT 2601, Australia, a phylogeny to estimate the geographical spread of orchids, evaluate the impor- 4Institute of Ecology and Biodiversity, tance of different regions in their diversification and assess the role of long-dis- Facultad de Ciencias, Universidad de Chile, tance dispersal (LDD) in generating orchid diversity. 5 Santiago, Chile, Department of Biology, Location Global. University of Florida, Gainesville, FL 32611, USA Methods Analyses use a phylogeny including species representing all five orchid subfamilies and almost all tribes and subtribes, calibrated against 17 angiosperm fossils. We estimated historical biogeography and assessed the