Demographic Parameters of Seabirds in the North Atlantic Along a Marine Productivity Gradient
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CT3 ARM.Docx
Summits on the Air – ARM for Madeira (CT3) Summits on the Air Madeira (CT3) Association Reference Manual Document Reference S84.1 Issue number 1.2 Date of issue 01-October-2015 Participation start date 01-October-2012 Authorised Date: 01-October-2012 on behalf of SOTA Management Team Association Manager Jose Luis Camacho CT3EE [email protected] Summits-on-the-Air an original concept by G3WGV and developed with G3CWI Notice: “Summits on the Air” SOTA and the SOTA logo are trademarks of the Programme. This document is copyright of the Programme. All other trademarks and copyrights referenced herein are acknowledged. Page 1 of 13 Document S84.1 Issue 1.2 Summits on the Air – ARM for Madeira (CT3) Table of contents Change Control ………………………………………………………………. 2 1.0 Association Reference Data .…………………………………………….…… 3 1.1 Programme derivation …………………………………………………….…… 3 1.2 General information …………………………………………………………… 4 1.3 Rights of way and access issues ………………………………………………. 4 1.4 Maps and navigation …………………………………………………………... 4 1.5 Safety considerations …………………………………………………………. 4 1.6 Foreign HAMs in Madeira ……………………………………………………. 5 1.7 Awards ………………………………………………………………………… 5 1.8 Disclaimer ……………………………………………………………………... 5 2.0 Summit reference data ………………………………………………………… 6 2.1 Region reference – Madeira Island …………………………………………… 6 2.1.1 Regional notes ………………………………………………………………… 6 2.1.2 Table of summits ……………………………………………………………… 6 2.2 Region reference - Porto Santos ………………………………………………. 7 2.2.1 Regional notes ………………………………………………………………… 7 2.2.2 Table of summits ……………………………………………………………… 7 2.3 Region reference - Ilhas Desertas ……………………………………………. 8 2.3.1 Regional notes ………………………………………………………………… 8 2.3.2 Table of summits ……………………………………………………………… 8 Change Control Date Version Details 01-Oct-12 1.0 First formal issue of this document 28-Jan-14 1.1 PS added, format cleaned up, typos corrected 01-Oct-15 1.2 Added the correct Association Reference Data chart for CT3 (deleted Poland data). -

Systematic List of the Birds
59.82(66.53) Article III.-THE MARINE ORNITHOLOGY OF THE CAPE VERDE ISLANDS, WITH A LIST OF ALL THE BIRDS OF THE ARCHIPELAGO BY ROBERT CUSHMAN MURPHY CONTENTS PAGE. INTRODUCTION........................................................... 211 HISTORICAL SKETCH OF CAPE VERDE ORNITHOLOGY .............. .......... 212 THE VISITS OF CORREIA AND THE AUTHOR.................................. 213 THE GEOGRAPHIC ENVIRONMENT....................... 216 OCEANOGRAPHY AND CLIMATE............................................ 221 GEOGRAPHIC RELATIONSHIPS OF THE BIRDS ............................... 226 BIBLIOGRAPHY......................................................... 232 SYSTEMATIC LIST OF THE BIRDS............................................ 233 INTRODUCTION The present paper is an indirect result of a brief visit which the writer made to the Cape Verde Islands in September 1912. It is more immediately linked, however, with a collecting trip undertaken during part of the spring and summer of 1922 by Mr. Jos6 Gongalves Correia, of New Bedford, Mass., whose specimens, numbering some three hundred skins of twenty species, have all been received by The American Museum of Natural History. As Correia's predominant object was to acquire adequate series of water birds, the terrestrial species of the Cape Verdes are but scantily represented in his collection, which is supplemented, to a certain extent, by a small number of specimens obtained by the writer in 1912. Notwithstanding the relatively limited material available for study, it has been thought advisable to include within this paper a systematic list of all the birds known from the Cape Verde Islands. The latest faunal contribution is that of Salvadori (1899). Not only have addi- tions to the avifauna been made since that date, but also forms new to science, including several endemic races, have been described. More- over, numerous nomenclatural changes made necessary because of the researches of Hartert ('V6gel der palaarktischen Fauna,' 1910-22) and other workers increase the need for a new list. -

FEEDING ECOLOGY of the CAPE VERDEAN SHEARWATER (Calonectris Edwardsii) POPULATION of RASO ISLET, CAPE VERDE (P1-B-11)
2nd World Seabird Conference “Seabirds: Global Ocean Sentinels” 26-30 October 2015 Cape Town, South Africa FEEDING ECOLOGY OF THE CAPE VERDEAN SHEARWATER (Calonectris edwardsii) POPULATION OF RASO ISLET, CAPE VERDE (P1-B-11) Isabel Rodrigues1,3; Nuno Oliveira 2 Rui Freitas 3 Tommy Melo1 Pedro Geraldes 2 1Biosfera I, Cabo Verde, www.biosfera1.com; 2SPEA, Portugal, www.spea.pt, 3 Universidade de Cabo Verde, www.unicv.edu.cv Raso Islet with only 5.76 km2, is an area of great importance to Cape Verdean Shearwaters as we find one of the largest colonies of the species there. The great biological value of this islet is even more remarkable for hosting very large populations of other species, such as the Brown Booby, Red-billed Tropicalbird and even the endemic Raso Lark, among others. Along with Branco Islet, also included in the Nature Reserve, both populations constitute about 75% of the nesting population of the Cape Verde islands (Fig. 1). The Cape Verde Shearwater (Procellariiformes, Procellariidae) (Fig. 2) is an endemic species of Cape Verde and has recently been separated from Calonectris diomedea species, due to their morphological and genetic differences, and pelagic habits; feeding mostly on the open sea (Hazevoet 1995). METHODOLOGY RESULTS The samples were collected between 14 October and 12 November, in two In total, 80 regurgitations from juvenile Cape Verde Shearwaters were collected; consecutive years, 2012 and 2013. We randomly obtained 80 samples of juvenile including 50 individuals sampled in 2012, and 30 individuals in 2013. regurgitation. Each juvenile was sampled only once. During or after handling, Based on knowledge of local fish populations and according to the current juveniles tend to regurgitate stomach contents without the need to resort to the description, the identified prey of Cape Verde shearwaters belonged to 5 species induced regurgitation method. -

Arachnida, Araneae) of Macaronesia II: the Native Forests and Dry Habitats of Madeira Archipelago (Madeira and Porto Santo Islands)
Biodiversity Data Journal 8: e47502 doi: 10.3897/BDJ.8.e47502 Data Paper Standardised inventories of spiders (Arachnida, Araneae) of Macaronesia II: The native forests and dry habitats of Madeira archipelago (Madeira and Porto Santo islands) Jagoba Malumbres-Olarte‡,§, Mário Boieiro ‡, Pedro Cardoso§,|,‡, Rui Carvalho ‡, Luís Carlos Fonseca Crespo¶,§, Rosalina Gabriel‡, Nuria Macías Hernández§,#, Octávio S. Paulo¤, Fernando Pereira‡, Carla Rego‡, Alejandra Ros-Prieto‡«, Isamberto Silva , Ana Vieira¤, François Rigal »,‡, Paulo A. V. Borges‡,˄ ‡ CE3C – Centre for Ecology, Evolution and Environmental Changes / Azorean Biodiversity Group and Universidade dos Açores, Angra do Heroísmo, Azores, Portugal § LIBRe – Laboratory for Integrative Biodiversity Research, Finnish Museum of Natural History, University of Helsinki, Helsinki, Finland | IUCN SSC Spider & Scorpion Specialist Group, Helsinki, Finland ¶ Biodiversity Research Institute UB, Departament Department of Evolutionary Biology, Ecology and Environmental Sciences (Arthropods), Barcelona, Spain # Island Ecology and Evolution Research Group, IPNA-CSIC, Tenerife, Canary Islands, Spain ¤ Faculdade de Ciências da Universidade de Lisboa, Departamento de Biologia Animal e Centro de Biologia Ambiental, Computational Biology and Population Genomics Group, Lisbon, Portugal « Instituto das Florestas e da Conservação da Natureza, Funchal, Portugal » Environment and Microbiology Team, Université de Pau et des Pays de l'Adour, Pau Cedex, France ˄ IUCN - SSC Mid-Atlantic Island Invertebrates Specialist -

10-11 April 2019
Strasbourg, 26 July 2019 T-PVS/DE (2019) 16 [de16e_2019.docx] CONVENTION ON THE CONSERVATION OF EUROPEAN WILDLIFE AND NATURAL HABITATS Standing Committee 40th meeting Strasbourg, 1-4 December 2020 __________ REPORT ON THE SPOT EXPERT APPRAISAL OF THE DESERTAS ISLANDS NATURE RESERVE (MADEIRA - PORTUGAL) 10-11 April 2019 Document prepared by Ms Blanca RAMOS (Spain) This document will not be distributed at the meeting. Please bring this copy. Ce document ne sera plus distribué en réunion. Prière de vous munir de cet exemplaire. T-PVS/DE (2019) 16 - 2 - Table of contents 1. INTRODUCTION .......................................................................................................................................... 3 2. BRIEF DESCRIPTION OF THE DESERTAS ISLANDS NATURE RESERVE ........................................ 5 3. EUROPEAN INTEREST OF THE SITE ....................................................................................................... 7 3.1. Fauna ..................................................................................................................................................... 7 3.2. Flora....................................................................................................................................................... 8 3.3. Exploitation of resources ....................................................................................................................... 9 3.4. European interest justifying the Diploma ............................................................................................. -

Oceans, Antarctica
G9102 ATLANTIC OCEAN. REGIONS, NATURAL FEATURES, G9102 ETC. .G8 Guinea, Gulf of 2950 G9112 NORTH ATLANTIC OCEAN. REGIONS, BAYS, ETC. G9112 .B3 Baffin Bay .B34 Baltimore Canyon .B5 Biscay, Bay of .B55 Blake Plateau .B67 Bouma Bank .C3 Canso Bank .C4 Celtic Sea .C5 Channel Tunnel [England and France] .D3 Davis Strait .D4 Denmark Strait .D6 Dover, Strait of .E5 English Channel .F45 Florida, Straits of .F5 Florida-Bahamas Plateau .G4 Georges Bank .G43 Georgia Embayment .G65 Grand Banks of Newfoundland .G7 Great South Channel .G8 Gulf Stream .H2 Halten Bank .I2 Iberian Plain .I7 Irish Sea .L3 Labrador Sea .M3 Maine, Gulf of .M4 Mexico, Gulf of .M53 Mid-Atlantic Bight .M6 Mona Passage .N6 North Sea .N7 Norwegian Sea .R4 Reykjanes Ridge .R6 Rockall Bank .S25 Sabine Bank .S3 Saint George's Channel .S4 Serpent's Mouth .S6 South Atlantic Bight .S8 Stellwagen Bank .T7 Traena Bank 2951 G9122 BERMUDA. REGIONS, NATURAL FEATURES, G9122 ISLANDS, ETC. .C3 Castle Harbour .C6 Coasts .G7 Great Sound .H3 Harrington Sound .I7 Ireland Island .N6 Nonsuch Island .S2 Saint David's Island .S3 Saint Georges Island .S6 Somerset Island 2952 G9123 BERMUDA. COUNTIES G9123 .D4 Devonshire .H3 Hamilton .P3 Paget .P4 Pembroke .S3 Saint Georges .S4 Sandys .S5 Smiths .S6 Southampton .W3 Warwick 2953 G9124 BERMUDA. CITIES AND TOWNS, ETC. G9124 .H3 Hamilton .S3 Saint George .S6 Somerset 2954 G9132 AZORES. REGIONS, NATURAL FEATURES, G9132 ISLANDS, ETC. .A3 Agua de Pau Volcano .C6 Coasts .C65 Corvo Island .F3 Faial Island .F5 Flores Island .F82 Furnas Volcano .G7 Graciosa Island .L3 Lages Field .P5 Pico Island .S2 Santa Maria Island .S3 Sao Jorge Island .S4 Sao Miguel Island .S46 Sete Cidades Volcano .T4 Terceira Island 2955 G9133 AZORES. -

Recent Bird Records from Fogo, Cape Verde Islands Rubén Baronea and Jens Heringb
Recent bird records from Fogo, Cape Verde Islands Rubén Baronea and Jens Heringb Observations récentes de Fogo, Îles du Cap-Vert. Des données sont présentées concernant 12 espèces d’oiseaux observées à Fogo, Îles du Cap-Vert, parmi lesquelles deux premières mentions pour l’île (Chevalier gambette Tringa totanus et Hirondelle de fenêtre Delichon urbicum), les premières données de nidification du Martinet du Cap-Vert Apus alexandri et les premières observations fiables du Phaéton à bec rouge Phaethon aethereus indiquant la nidification probable de celui-ci. Des informations sont également présentées sur d’autres taxons mal connus à Fogo, tels que certaines espèces pélagiques et l’Effraie des clochers Tyto alba detorta. Summary. We present data on 12 bird species observed on Fogo, Cape Verde Islands, among them two first records for the island (Common Redshank Tringa totanus and Common House Martin Delichon urbicum), the first breeding records of Cape Verde Swift Apus alexandri and the first reliable observations of Red-billed Tropicbird Phaethon aethereus indicating probable breeding. Information on other taxa poorly known on Fogo, such as some pelagic seabirds and Barn Owl Tyto alba detorta, is also given. ogo, one of the Cape Verde Islands, is situated reported previously, and some others for which F in the leeward group (‘Ilhas do Sotavento’), there are only a limited number of observations. c.724 km from the African continent. With Local information on breeding birds was mainly a surface area of 478 km2, the highest peak collected by RB. Dates of our visits are as follows: (Pico Novo) reaches 2,829 m (Michell-Thomé 18–21 October 2004 (JH & H. -
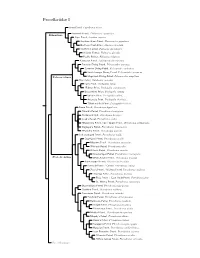
Procellariidae Species Tree
Procellariidae I Snow Petrel, Pagodroma nivea Antarctic Petrel, Thalassoica antarctica Fulmarinae Cape Petrel, Daption capense Southern Giant-Petrel, Macronectes giganteus Northern Giant-Petrel, Macronectes halli Southern Fulmar, Fulmarus glacialoides Atlantic Fulmar, Fulmarus glacialis Pacific Fulmar, Fulmarus rodgersii Kerguelen Petrel, Aphrodroma brevirostris Peruvian Diving-Petrel, Pelecanoides garnotii Common Diving-Petrel, Pelecanoides urinatrix South Georgia Diving-Petrel, Pelecanoides georgicus Pelecanoidinae Magellanic Diving-Petrel, Pelecanoides magellani Blue Petrel, Halobaena caerulea Fairy Prion, Pachyptila turtur ?Fulmar Prion, Pachyptila crassirostris Broad-billed Prion, Pachyptila vittata Salvin’s Prion, Pachyptila salvini Antarctic Prion, Pachyptila desolata ?Slender-billed Prion, Pachyptila belcheri Bonin Petrel, Pterodroma hypoleuca ?Gould’s Petrel, Pterodroma leucoptera ?Collared Petrel, Pterodroma brevipes Cook’s Petrel, Pterodroma cookii ?Masatierra Petrel / De Filippi’s Petrel, Pterodroma defilippiana Stejneger’s Petrel, Pterodroma longirostris ?Pycroft’s Petrel, Pterodroma pycrofti Soft-plumaged Petrel, Pterodroma mollis Gray-faced Petrel, Pterodroma gouldi Magenta Petrel, Pterodroma magentae ?Phoenix Petrel, Pterodroma alba Atlantic Petrel, Pterodroma incerta Great-winged Petrel, Pterodroma macroptera Pterodrominae White-headed Petrel, Pterodroma lessonii Black-capped Petrel, Pterodroma hasitata Bermuda Petrel / Cahow, Pterodroma cahow Zino’s Petrel / Madeira Petrel, Pterodroma madeira Desertas Petrel, Pterodroma -

Shearwatersrefs V1.10.Pdf
Introduction I have endeavoured to keep typos, errors, omissions etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2018 & 2019 to: [email protected]. Please note that this and other Reference Lists I have compiled are not exhaustive and are best employed in conjunction with other sources. Grateful thanks to Ashley Fisher (www.scillypelagics.com) for the cover images. All images © the photographer. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds.) 2017. IOC World Bird List. Available from: http://www.worldbirdnames.org/ [version 7.3 accessed August 2017]). Version Version 1.10 (January 2018). Cover Main image: Great Shearwater. At sea 3’ SW of the Bishop Rock, Isles of Scilly. 8th August 2009. Picture by Ashley Fisher. Vignette: Sooty Shearwater. At sea off the Isles of Scilly. 14th August 2009. Picture by Ashley Fisher. Species Page No. Audubon's Shearwater [Puffinus lherminieri] 34 Balearic Shearwater [Puffinus mauretanicus] 28 Bannerman's Shearwater [Puffinus bannermani] 37 Barolo Shearwater [Puffinus baroli] 38 Black-vented Shearwater [Puffinus opisthomelas] 30 Boyd's Shearwater [Puffinus boydi] 38 Bryan's Shearwater [Puffinus bryani] 29 Buller's Shearwater [Ardenna bulleri] 15 Cape Verde Shearwater [Calonectris edwardsii] 12 Christmas Island Shearwater [Puffinus nativitatis] 23 Cory's Shearwater [Calonectris borealis] 9 Flesh-footed Shearwater [Ardenna carneipes] 21 Fluttering -

Threats to Seabirds: a Global Assessment 2 3 4 Authors: Maria P
1 Threats to seabirds: a global assessment 2 3 4 Authors: Maria P. Dias1*, Rob Martin1, Elizabeth J. Pearmain1, Ian J. Burfield1, Cleo Small2, Richard A. 5 Phillips3, Oliver Yates4, Ben Lascelles1, Pablo Garcia Borboroglu5, John P. Croxall1 6 7 8 Affiliations: 9 1 - BirdLife International. The David Attenborough Building, Pembroke Street Cambridge CB2 3QZ UK 10 2 - BirdLife International Marine Programme, RSPB, The Lodge, Sandy, SG19 2DL 11 3 – British Antarctic Survey. Natural Environment Research Council, High Cross, Madingley Road, 12 Cambridge CB3 0ET, UK 13 4 – Centre for the Environment, Fishery and Aquaculture Science, Pakefield Road, Lowestoft, NR33, UK 14 5 - Global Penguin Society, University of Washington and CONICET Argentina. Puerto Madryn U9120, 15 Chubut, Argentina 16 * Corresponding author: Maria Dias, [email protected]. BirdLife International. The David 17 Attenborough Building, Pembroke Street Cambridge CB2 3QZ UK. Phone: +44 (0)1223 747540 18 19 20 Acknowledgements 21 We are very grateful to Bartek Arendarczyk, Sophie Bennett, Ricky Hibble, Eleanor Miller and Amy 22 Palmer-Newton for assisting with the bibliographic review. We thank Rachael Alderman, Pep Arcos, 23 Jonathon Barrington, Igor Debski, Peter Hodum, Gustavo Jimenez, Jeff Mangel, Ken Morgan, Paul Sagar, 24 Peter Ryan, and other members of the ACAP PaCSWG, and the members of IUCN SSC Penguin Specialist 25 Group (Alejandro Simeone, Andre Chiaradia, Barbara Wienecke, Charles-André Bost, Lauren Waller, Phil 26 Trathan, Philip Seddon, Susie Ellis, Tom Schneider and Dee Boersma) for reviewing threats to selected 27 species. We thank also Andy Symes, Rocio Moreno, Stuart Butchart, Paul Donald, Rory Crawford, 28 Tammy Davies, Ana Carneiro and Tris Allinson for fruitful discussions and helpful comments on earlier 29 versions of the manuscript. -

Trip Report Cape Verde
Cape Verde March 17 th – 24 th 2018 Cape Verde Swamp Warbler Red-billed Tropicbird Raso Lark Islands (and sites) visited: Santiago Praia cliffs, Praia plains, Barragem de Poilão, Pedra Badejo lagoons, Barragem de Figuera Gorda, Jardim Botanico Boavista Sal Rei harbor, Rabil lagoon, Ponta da Varandinha, Zone Humide de Lecacão, Curral Velho, centre of isle São Nicolau Ponta do Barril, Taraffal Raso General Information Getting there: Cape Verde lies approx. 600 km west of Senegal and consists of 9 inhabited plus a number of un- inhabited islands. Thus flying there is the most common way to reach Cape Verde. Most visitors will be landing on Sal or Boavista as these are the most touristic islands and several airlines go there. Santiago and Sao Vincente also have international airports. TAP heads there via Lisbon. A visa is required for EU-citizens which can be obtained in the arrival airport for 27€. This proce- dure requires some time, depending on the amount of passengers in the respective plane. Visas can also be requested in the Cape Verde embassies in most countries. This was 45€ for Germany. Getting around: Between several islands: If you want to visit more than one island the local airline Binter offers flights for reasonable prices. They are said to not always stick to the schedule but were very reli- able in my case. Some islands can be reached by ferry but be aware that during the windy season in winter and spring the sea can be very rough and many people get seasick. On the islands: This proves to be one of the expensive parts. -

Study and Conservation of Breeding Seabirds in Rabo-De-Junco Islet Sal Island, Cape Verde
Associação Projeto Biodiversidade (Project Biodiversity) Albert Taxonera & Marcos Hernández Study and Conservation of breeding Seabirds in Rabo-de-junco Islet Sal Island, Cape Verde Project Biodiveristy Project Biodiversity is a Cabo Verdean organization committed to conserving and restoring the island’s unique ecosystems. Based on the island of Sal, the project implements community- based initiatives that promote conservation and better understanding of the island’s natural resources while increasing economic opportunities for the growing local community. Visit www.projectbiodiversity.org to know more about our initiatives. Seabird species of Sal Cabo Verde is a volcanic archipelago of 10 island and several islets included in the Macaronesia region, and located over 570 km off the coast of West Africa. The Island of Sal is one of the nine inhabited islands and located in the northeast corner of the archipelago (Figure 1). It is fairly flat with an area of 216 km2 and its highest point culminates at 408 meters. The southern part of the island, where sandy beaches extend, concentrates most of the touristic development, which is the main economic driver of the country, contributing to 18% of the national GDP. Figure 1 – Location of the Cabo Verde archipelago and Sal island as one of its 10 islands. Cabo Verde’s archipelago is an important nesting site for seabirds, with 8 different breeding species. From those, three are endemic species from the archipelago, the Cape Verde Petrel (Pterodroma feae), the Cape Verde Shearwater (Calonectris edwardsii) and the Cape Verde Storm-Petrel (Hydrobates jabejabe). There are also 2 endemic subspecies, the Cape Verde Little Shearwater (Puffinus lherminieri boydi) and the White-faced Storm-Petrel (Pelagodroma marina eadesorum).