Assessment of Efficient and Sustainable Tools for Cholera Detection and Intervention in Low Resource Settings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GE84/275 BR IFIC Nº 2893 Section Spéciale Special Section
Section spéciale Index BR IFIC Nº 2893 Special Section GE84/275 Sección especial Indice International Frequency Information Circular (Terrestrial Services) ITU - Radiocommunication Bureau Circular Internacional de Información sobre Frecuencias (Servicios Terrenales) UIT - Oficina de Radiocomunicaciones Circulaire Internationale d'Information sur les Fréquences (Services de Terre) UIT - Bureau des Radiocommunications Date/Fecha : 16.04.2019 Expiry date for comments / Fecha limite para comentarios / Date limite pour les commentaires : 25.07.2019 Description of Columns / Descripción de columnas / Description des colonnes Intent Purpose of the notification Propósito de la notificación Objet de la notification 1a Assigned frequency Frecuencia asignada Fréquence assignée 4a Name of the location of Tx station Nombre del emplazamiento de estación Tx Nom de l'emplacement de la station Tx B Administration Administración Administration 4b Geographical area Zona geográfica Zone géographique 4c Geographical coordinates Coordenadas geográficas Coordonnées géographiques 6a Class of station Clase de estación Classe de station 1b Vision / sound frequency Frecuencia de portadora imagen/sonido Fréquence image / son 1ea Frequency stability Estabilidad de frecuencia Stabilité de fréquence 1e carrier frequency offset Desplazamiento de la portadora Décalage de la porteuse 7c System and colour system Sistema de transmisión / color Système et système de couleur 9d Polarization Polarización Polarisation 13c Remarks Observaciones Remarques 9 Directivity Directividad -

Cameroon Humanitarian Situation Report
Cameroon Humanitarian Situation Report ©UNICEF Cameroon/2019 SITUATION IN NUMBERS Highlights August 2019 2,300,000 • More than 118,000 people have benefited from UNICEF’s # of children in need of humanitarian assistance humanitarian assistance in the North-West and South-West 4,300,000 regions since January including 15,800 in August. # of people in need (Cameroon Humanitarian Needs Overview 2019) • The Rapid Response Mechanism (RRM) strategy, Displacement established in the South-West region in June, was extended 530,000 into the North-West region in which 1,640 people received # of Internally Displaced Persons (IDPs) in the North- WASH kits and Long-Lasting Insecticidal Nets (LLINs) in West and South-West regions (OCHA Displacement Monitoring, July 2019) August. 372,854 # of IDPs and Returnees in the Far-North region • In August, 265,694 children in the Far-North region were (IOM Displacement Tracking Matrix 18, April 2019) vaccinated against poliomyelitis during the final round of 105,923 the vaccination campaign launched following the polio # of Nigerian Refugees in rural areas (UNHCR Fact Sheet, July 2019) outbreak in May. UNICEF Appeal 2019 • During the month of August, 3,087 children received US$ 39.3 million psychosocial support in the Far-North region. UNICEF’s Response with Partners Total funding Funds requirement received Sector Total UNICEF Total available 20% $ 4.5M Target Results* Target Results* Carry-over WASH: People provided with 374,758 33,152 75,000 20,181 $ 3.2 M access to appropriate sanitation 2019 funding Education: Number of boys and requirement: girls (3 to 17 years) affected by 363,300 2,415 217,980 0 $39.3 M crisis receiving learning materials Nutrition**: Number of children Funding gap aged 6-59 months with SAM 60,255 39,727 65,064 40,626 $ 31.6M admitted for treatment Child Protection: Children reached with psychosocial support 563,265 160,423 289,789 87,110 through child friendly/safe spaces C4D: Persons reached with key life- saving & behaviour change 385,000 431,034 messages *Total results are cumulative. -

Cameroon |Far North Region |Displacement Report Round 15 | 03– 15 September 2018
Cameroon |Far North Region |Displacement Report Round 15 | 03– 15 September 2018 Cameroon | Far North Region | Displacement Report | Round 15 | 03– 15 September 2018 The opinions expressed in the report are those of the authors and do not necessarily reflect the views of the International Organization for Migration (IOM). The designations employed and the presentation of material throughout the report do not imply the expression of any opinion whatsoever on the part of IOM concerning the legal status of any country, territory, city or area, or of its authorities, or concerning its frontiers or boundaries1. IOM is committed to the principle that humane and orderly migration benefits migrants and society. As an intergovernmental organization, IOM acts with its partners in the international community to assist in meeting the operational challenges of migration, advance understanding of migration issues, encourage social and economic development through migration and uphold the human dignity and well-being of migrants. International Organization for Migration Cameroon Mission Maroua Sub-Office UN House Comice Maroua Far North Region Cameroon Tel.: +237 222 20 32 78 E-mail: [email protected] Websites: https://ww.iom.int/fr/countries/cameroun and https://displacement.iom.int/cameroon All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the publisher. 1The maps included in this report are illustrative. The representations and the use of borders and geographic names may include errors and do not imply judgment on legal status of territories nor acknowledgement of borders by the Organization. -

CAMEROON : Far North - Idps Overview 29 March 2015
CAMEROON : Far North - IDPs Overview 29 March 2015 Overview from the region Total IDP Population 96,042 Age & Gender Violent attacks by Boko Haram in Nigeria as well as in villages along the border inside Cameroon have increased during the last months. Reports by those who fled Age (Years) Male Female indicate lootings of villages, killings, mass execution, and kidnappings including of children which resulted in large scale displacement. The vast majority of the IDPs 0-4 8% 7% are reportedly staying with host communities. Latest figures shared by the 5-10 7% 7% Government with UNHCR indicate a total of 96,042 IDPs in three departments 11-14 6% 6% (Logone et Chari: 39,853; Maya Tsanaga: 29,200; Mayo Sava : 26,989). To provide timely protection and assistance to IDPs and host communities the Government 15-19 5% 5% asked UNHCR to engage in a profiling exercise which is currently underway. First 20-29 9% 9% results of the UNHCR protection assessment will be available at the end of the 30-44 8% 8% week. 49-59 4% 5% FOOD & NUTRITION 39,853 60+ 2% 3% SAM prevalence (6-59 Months) EDUCATION 120 schools were forced to close in 10 districts of the Far North for the current 2 % accademic year (2014-2015) 0% 1% 2% 3% 4% 5% 33,163 children (43% girls) are out of school or have been forced to seek access 26,989 to schooling outside of their native communities as a result of school closures in The Far North region has a prevalence of severe acute malnutrition (SAM) of 2.0% affected districts corresponding to the emergency threshold. -

CURICULUMVITAE JOANNE KATZ PERSONAL DATA Home
C U R I C U L U M V I T A E JOANNE KATZ PERSONAL DATA Home: 2102 South Road Baltimore, Maryland 21209 Phone: (410) 466-4905 Office: Global Disease Epidemiology and Control Program Department of International Health Johns Hopkins University Bloomberg School of Public Health Room E5527, 615 N. Wolfe Street Baltimore, Maryland 21205-2103 Phone: (410) 955-7016 Fax: (410) 955-2029 email: [email protected] EDUCATION Sc.D./1993 Johns Hopkins University, Bloomberg School of Public Health, Baltimore, Maryland International Health M.S./1982 Princeton University, Princeton, New Jersey Mathematical Statistics B.Sc./1978 University of Cape Town, Cape Town, South Africa Mathematical Statistics, Economics Curriculum Vitae 2 Joanne Katz PROFESSIONAL EXPERIENCE Joint Appointments in: Professor Department of International Health Dept. of Biostatistics (1984 - present) Bloomberg School of Public Health Bloomberg School of Public Health Johns Hopkins University Johns Hopkins University 1997 - present Dept. of Ophthalmology (1994 - present) Associate Chair and Director School of Medicine Academic Programs Johns Hopkins University Department of International Health School of Medicine 2013 – present Johns Hopkins University Associate Chair and Director Dept. of Epidemiology (1996 - present) Global Disease Epidemiology and Control Program Bloomberg School of Public Health Department of International Health Johns Hopkins University 2006 – 2013 Deputy Director Global Disease Epidemiology and Control Program Department of International Health 2002 – 2006 Center Appointments Center for Global Health (2006 - present) International Center for Maternal and Neonatal Health (2006 – present) Center for Human Nutrition (1996 - present) Center for Clinical Trials (1994 - present) Dana Center for Preventive Ophthalmology (1982 - present) Visiting Professor (August 2003) Institute for International Health University of Sydney, New South Wales, Australia. -

Overview of Human Wildlife Conflict in Cameroon
Overview of Human Wildlife Conflict in Cameroon Poto: Etoga Gilles WWF Campo, 2011 By Antoine Justin Eyebe Guy Patrice Dkamela Dominique Endamana POVERTY AND CONSERVATION LEARNING GROUP DISCUSSION PAPER NO 05 February 2012 Contents EXECUTIVE SUMMARY ................................................................................................................ 3 1. Introduction ......................................................................................................................... 4 2. Evidence of HWC in Cameroon............................................................................................ 4 3. Typology of HWC ................................................................................................................. 7 3.1 Crop destruction ........................................................................................................... 7 3.2 Attacks on domestic animals ..................................................................................... 10 3.3 Human death, injuries and damage to property ....................................................... 11 4. The Policy and Institutional Framework for Human-Wildlife Conflict Management at the State level in Cameroon ............................................................................................................ 11 4.1. Statutory instruments and institutions for HWC management ................................. 12 4.1.1 Protection of persons and property against animals ......................................... 12 4.1.2 Compensation -

Proceedingsnord of the GENERAL CONFERENCE of LOCAL COUNCILS
REPUBLIC OF CAMEROON REPUBLIQUE DU CAMEROUN Peace - Work - Fatherland Paix - Travail - Patrie ------------------------- ------------------------- MINISTRY OF DECENTRALIZATION MINISTERE DE LA DECENTRALISATION AND LOCAL DEVELOPMENT ET DU DEVELOPPEMENT LOCAL Extrême PROCEEDINGSNord OF THE GENERAL CONFERENCE OF LOCAL COUNCILS Nord Theme: Deepening Decentralization: A New Face for Local Councils in Cameroon Adamaoua Nord-Ouest Yaounde Conference Centre, 6 and 7 February 2019 Sud- Ouest Ouest Centre Littoral Est Sud Published in July 2019 For any information on the General Conference on Local Councils - 2019 edition - or to obtain copies of this publication, please contact: Ministry of Decentralization and Local Development (MINDDEVEL) Website: www.minddevel.gov.cm Facebook: Ministère-de-la-Décentralisation-et-du-Développement-Local Twitter: @minddevelcamer.1 Reviewed by: MINDDEVEL/PRADEC-GIZ These proceedings have been published with the assistance of the German Federal Ministry for Economic Cooperation and Development (BMZ) through the Deutsche Gesellschaft für internationale Zusammenarbeit (GIZ) GmbH in the framework of the Support programme for municipal development (PROMUD). GIZ does not necessarily share the opinions expressed in this publication. The Ministry of Decentralisation and Local Development (MINDDEVEL) is fully responsible for this content. Contents Contents Foreword ..............................................................................................................................................................................5 -
Displacement Tracking Matrix I Dtm
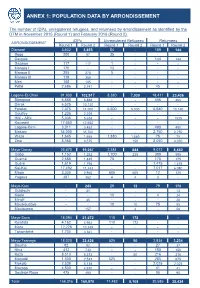
ANNEX 1: POPULATION DATA BY ARRONDISSEMENT The number of IDPs, unregistered refugees, and returnees by arrondissement as identified by the DTM in November 2015 (Round 1) and February 2016 (Round 2). IDPs Unregistered Refugees Returnees ARRONDISSEMENT Round 1 Round 2 Round 1 Round 2 Round 1 Round 2 Diamaré 3,602 3,655 54 - 189 144 Bogo 200 57 35 - - - Dargala - - - - 144 144 Gazawa 117 117 1 - - - Maroua I 170 - 13 - - - Maroua II 205 270 5 - - - Maroua III 119 365 - - - - Meri 105 105 - - - - Pétté 2,686 2,741 - - 45 - Logone-Et-Chari 91,930 102,917 8,380 7,030 18,411 23,436 Blangoua 6,888 6,888 - - 406 406 Darak 6,525 10,120 - - - - Fotokol 7,375 11,000 6,500 5,000 6,640 10,140 Goulfey 1,235 2,229 - - - - Hilé - Alifa 5,036 5,638 - - - 1525 Kousséri 17,650 17,650 - - - - Logone-Birni 3,011 3,452 - - 490 490 Makary 34,000 35,700 - - 2,750 2,750 Waza 1,645 1,665 1,880 1,880 75 75 Zina 8,565 8,575 - 150 8,050 8,050 Mayo-Danay 26,670 19,057 2,384 844 9,072 8,450 Gobo 1,152 1,252 1,700 235 390 595 Guémé 2,685 1,445 75 - 175 175 Guéré 1,619 1,795 - - 1,475 1,475 Kai-Kai 17,492 11,243 - - 7,017 6,080 Maga 3,335 2,960 605 605 12 125 Yagoua 387 362 4 4 3 - Mayo-Kani - 243 29 12 79 170 Guidiguis - 41 - 13 Kaélé - - 19 - 4 24 Mindif - 45 - - - 20 Moulvoudaye - - 10 10 75 55 Moutourwa - 157 - 2 - 58 Mayo-Sava 18,094 21,672 110 172 - - Kolofata 4,162 4,962 110 172 - - Mora 12,228 13,349 - - - - Tokombéré 1,704 3,361 - - - - Mayo-Tsanaga 18,020 22,426 525 50 2,834 3,234 Bourha 82 92 - - 27 37 Hina 412 730 - - 150 150 Koza 8,513 8,513 - 50 216 216 Mogodé 1,500 2,533 525 - 285 675 Mokolo 2,538 5,138 - - 2,025 2,025 Mozogo 4,500 4,930 - - 41 41 Soulèdé-Roua 475 490 - - 90 90 TotalDTM Cameroon 158,316 Round169,970 1 – November 11,482 2015 8,108 30,585 35,434 1 ANNEXE 2: LOCATIONS OF DISPLACED INDIVIDUALS Location of internally displaced persons, unregistered refugees, and returnees in the Far North region. -

Cameroon - Food Insecurity Experience Scale (FIES)
National Data Archive Cameroon - Food Insecurity Experience Scale (FIES) FAO Statistics Division Report generated on: July 3, 2019 Visit our data catalog at: https://microdata.fao.org/index.php 1 Cameroon - Food Insecurity Experience Scale (FIES) Overview Identification ID NUMBER CMR_2016_FIES_v01_EN_M_v01_A_OCS Overview ABSTRACT Sustainable Development Goal (SDG) target 2.1 commits countries to end hunger, ensure access by all people to safe, nutritious and sufficient food all year around. Indicator 2.1.2, “Prevalence of moderate or severe food insecurity based on the Food Insecurity Experience Scale (FIES)”, provides internationally-comparable estimates of the proportion of the population facing difficulties in accessing food. More detailed background information is available at http://www.fao.org/in-action/voices-of-the-hungry/fies/en/ . The FIES-based indicators are compiled using the FIES survey module, containing 8 questions. Two indicators can be computed: 1. The proportion of the population experiencing moderate or severe food insecurity (SDG indicator 2.1.2), 2. The proportion of the population experiencing severe food insecurity. These data were collected by FAO through the Gallup World Poll. General information on the methodology can be found here: https://www.gallup.com/178667/gallup-world-poll-work.aspx. National institutions can also collect FIES data by including the FIES survey module in nationally representative surveys. Microdata can be used to calculate the indicator 2.1.2 at national level. Instructions for computing this indicator are described in the methodological document available under the "DOCUMENTATION" tab above. Disaggregating results at sub- national level is not encouraged because estimates will suffer from substantial sampling and measurement error. -

Cmr Extreme-Nord A0 V1.2 En.Pdf
- Extrême-Nord Region Cameroon 15°E HAm-Doback Kom adu gu KANEM Yobe HKangalom HDoum-Doum LAC !Baga L a c T c h a d HKouloudia "Massakory ! Kukawa H HTourba HADJER-LAMIS Darak Karal Ngoum H ! Tchika ! Blangoua H Karéna Mani ! D2 H Hilé-Alifa H ! D2 Ngarkawa ! Magala Kabir Monguno ! Manawadji Cha ! Ngouma ri ! ! Fadjé Nadji D2 ! ! ! D 1 Dougoumsilio II H D1 Sagmé Bomboyo ! Makary ! Amchilga ! 12°30'N Ngaiwa ! 12°30'N Woulki NDJAMENA ! ) Kaoussé ! ! Kokio D2 Massaguet Dororoya D 1a ! ! D 1 Guéilala Biamo HN'Djamena-Fara ! Digam Fotokol ! H Gamboru! H N1A Bodo Nimia Goulfey ! Amsabang ! ! ! Ngala ! Blamé Mafoufou ! Ngonféla Rann ! ! N1A Sagmé ! Afadé ! ! Ngardoukoum Amfara ! Maltam ! ! N'Djaména BORNO Ngréé \! N1 C H A D N 1 Kousséri Linia ! H Dikwa Maï-ache L O G O N E - H HKoundoul E T - ! Dabanga C H A R I CHARI-BAGUIRMI !Maiduguri N 1 Logone-Birni H N I G E R I A )Mandalia !Banki Lougoun Hinalé H N 1 ! !Bama !Kajeri HLa-Loumia H Waza Boundéri ! Omaka ! N 1 Zina H Am Chidé !Banki ! ! Limani ! M AY O - Kerawa Pulka ! D9 Magdémé S AVA Kolofata ! N14 H ! Gansé ! Kossa Ashigashiya N14 ! ! ! Kourgui D3 Charif-Moussari ! Doulo !Gwoza Djoundé Geldavi ! ! Mora Ch ! Alagarno ar Kouyapé ! i ! Zélévet ! Méké Malika ! ! Mémé Moskota Mozogo ! ! H Baldama Warba H National capital Zouetva ! ! Petté Ouldémé ! Region capital Tourou Ouzal ! Fadaré ! ! Gaboua ! Guelendeng ! Palbara ) Department capital Koza Mouyengué ! H Makilingai D I A M A R É Arrondissement capital H ! Djingliya ! Balda Maga Pouss ! Kilda Seraoua ! H ! H ! Djiddel Region limit Tokombéré -

MCW 2020 HOF & WOT Their Legacy, Our Future
Maryland Women’s Hall of Fame and Maryland Women of Tomorrow 2020 Induction and Awards Ceremony Valiant Women Their Legacy, Our Future Thursday, March 19, 2020 4 p.m. Ceremony Conference Room East 6 p.m. Reception Conference Room West Miller Senate Office Building Annapolis, Maryland Presented by Maryland Commission for Women The Women Legislators of the Maryland General Assembly, Inc. Maryland Department of Human Services The Foundation for the Maryland Commission for Women, Inc. Maryland Commission for Women 51 Monroe Street, Suite 1034 Rockville, MD 20850 301-610-4523 www.marylandwomen.org The Maryland Commission for Women was first established in 1965 and was set in state law in 1971. An office in the Department of Human Resources, the Commission is a 25-member advisory board whose duties outlined in its enabling legislation include: study the status of women in our state, recommend methods of overcoming discrimination, recognize women’s accomplishments and contributions, and provide informed advice to the executive and legislative branches of government on the issues concerning the women of our state. Commissioners are appointed to four-year terms by the Governor, with confirmation by the Maryland State Senate, and serve without compensation. Officers Yun Jung Yang, Chair Yun Jung Yang, Esq., Chair, Montgomery County Tawanda A. Bailey, First Vice Chair, Frederick County Carole Jaar Sepe, Second Vice Chair, Frederick County Commissioners: Tammy Bresnahan, Anne Arundel County Bonnie Nelson Luna, Wicomico County Deborah L. Cartee, Anne Arundel County Brenda J. McChriston, Howard County Gloria Chang, Montgomery County Ishrat N. Memon, Howard County Maureen Colburn, Montgomery County April Nyman, Anne Arundel County Amanda L. -

241 Cameroon
Cameroon: Confronting Boko Haram $IULFD5HSRUW1 _ 1RYHPEHU 7UDQVODWLRQIURP)UHQFK +HDGTXDUWHUV ,QWHUQDWLRQDO&ULVLV*URXS $YHQXH/RXLVH %UXVVHOV%HOJLXP 7HO )D[ EUXVVHOV#FULVLVJURXSRUJ Table of Contents Executive Summary ................................................................................................................... i Recommendations..................................................................................................................... iii I. Introduction ..................................................................................................................... 1 II. Far North: History of a Vulnerable Region ...................................................................... 2 A. Far North: Between Violence and Smuggling ........................................................... 2 B. A Region Vulnerable to Infiltration by Boko Haram ................................................. 3 1. Dismal socio-economic indicators and absence of the state ................................ 3 2. A Sufi Islamic tradition under competitive pressure ........................................... 5 III. Boko Haram’s Infiltration into Far North ........................................................................ 8 A. Boko Haram Sinks Local Roots ................................................................................. 8 1. 2004-2013: early traces evolve into an established presence .............................. 8 2. 2014-2016: overt conflict ....................................................................................