Resource and Habitat Use of Northern Bottlenose Whales in the Gully: Ecology, Diving and Ranging Behaviour
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of Southern Ocean Squids Using Nets and Beaks
Marine Biodiversity (2020) 50:98 https://doi.org/10.1007/s12526-020-01113-4 REVIEW A review of Southern Ocean squids using nets and beaks Yves Cherel1 Received: 31 May 2020 /Revised: 31 August 2020 /Accepted: 3 September 2020 # Senckenberg Gesellschaft für Naturforschung 2020 Abstract This review presents an innovative approach to investigate the teuthofauna from the Southern Ocean by combining two com- plementary data sets, the literature on cephalopod taxonomy and biogeography, together with predator dietary investigations. Sixty squids were recorded south of the Subtropical Front, including one circumpolar Antarctic (Psychroteuthis glacialis Thiele, 1920), 13 circumpolar Southern Ocean, 20 circumpolar subantarctic, eight regional subantarctic, and 12 occasional subantarctic species. A critical evaluation removed five species from the list, and one species has an unknown taxonomic status. The 42 Southern Ocean squids belong to three large taxonomic units, bathyteuthoids (n = 1 species), myopsids (n =1),andoegopsids (n = 40). A high level of endemism (21 species, 50%, all oegopsids) characterizes the Southern Ocean teuthofauna. Seventeen families of oegopsids are represented, with three dominating families, onychoteuthids (seven species, five endemics), ommastrephids (six species, three endemics), and cranchiids (five species, three endemics). Recent improvements in beak identification and taxonomy allowed making new correspondence between beak and species names, such as Galiteuthis suhmi (Hoyle 1886), Liguriella podophtalma Issel, 1908, and the recently described Taonius notalia Evans, in prep. Gonatus phoebetriae beaks were synonymized with those of Gonatopsis octopedatus Sasaki, 1920, thus increasing significantly the number of records and detailing the circumpolar distribution of this rarely caught Southern Ocean squid. The review extends considerably the number of species, including endemics, recorded from the Southern Ocean, but it also highlights that the corresponding species to two well-described beaks (Moroteuthopsis sp. -

Trace Element Analysis Reveals Bioaccumulation in the Squid Gonatus Fabricii from Polar Regions of the Atlantic Ocean A
Trace element analysis reveals bioaccumulation in the squid Gonatus fabricii from polar regions of the Atlantic Ocean A. Lischka, T. Lacoue-Labarthe, P. Bustamante, U. Piatkowski, H.J.T. Hoving To cite this version: A. Lischka, T. Lacoue-Labarthe, P. Bustamante, U. Piatkowski, H.J.T. Hoving. Trace element analysis reveals bioaccumulation in the squid Gonatus fabricii from polar regions of the Atlantic Ocean. Envi- ronmental Pollution, Elsevier, 2020, 256, pp.113389. 10.1016/j.envpol.2019.113389. hal-02412742 HAL Id: hal-02412742 https://hal.archives-ouvertes.fr/hal-02412742 Submitted on 15 Dec 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Trace element analysis reveals bioaccumulation in the squid Gonatus fabricii from polar regions of the Atlantic Ocean A. Lischka1*, T. Lacoue-Labarthe2, P. Bustamante2, U. Piatkowski3, H. J. T. Hoving3 1AUT School of Science New Zealand, Auckland University of Technology, Private Bag 92006, 1142, Auckland, New Zealand 2 Littoral Environnement et Sociétés (LIENSs), UMR 7266 CNRS-La Rochelle Université, 2 rue Olympe de Gouges, 17000 La Rochelle, France 3 GEOMAR, Helmholtz Centre for Ocean Research Kiel, Düsternbrooker Weg 20, 24105 Kiel, Germany *corresponding author: [email protected] 1 Abstract: The boreoatlantic gonate squid (Gonatus fabricii) represents important prey for top predators—such as marine mammals, seabirds and fish—and is also an efficient predator of crustaceans and fish. -

Fish Bulletin 152. Food Habits of Albacore, Bluefin Tuna, and Bonito in California Waters
UC San Diego Fish Bulletin Title Fish Bulletin 152. Food Habits of Albacore, Bluefin Tuna, and Bonito In California Waters Permalink https://escholarship.org/uc/item/7t5868rd Authors Pinkas, Leo Oliphant, Malcolm S Iverson, Ingrid L.K. Publication Date 1970-06-01 eScholarship.org Powered by the California Digital Library University of California STATE OF CALIFORNIA THE RESOURCES AGENCY DEPARTMENT OF FISH AND GAME FISH BULLETIN 152 Food Habits of Albacore, Bluefin Tuna, and Bonito In California Waters By Leo Pinkas , Malcolm S. Oliphant, and Ingrid L. K. Iverson 1971 1 2 ABSTRACT The authors investigated food habits of albacore, Thunnus alalunga, bluefin tuna, Thunnus thynnus, and bonito, Sarda chiliensis, in the eastern North Pacific Ocean during 1968 and 1969. While most stomach samples came from fish caught commercially off southern California and Baja California, some came from fish taken in central Califor- nia, Oregon, and Washington waters. Standard procedures included enumeration of food items, volumetric analysis, and measure of frequency of occur- rence. The authors identified the majority of forage organisms to the specific level through usual taxonomic methods for whole animals. Identification of partially digested animals was accomplished through the use of otoliths for fish, beaks for cephalopods, and the exoskeleton for invertebrates. A pictorial guide to beaks of certain eastern Pacific cephalopods was prepared and proved helpful in identifying stomach contents. This guide is presented in this publication. The study indicates the prominent forage for bluefin tuna, bonito, and albacore in California waters is the northern anchovy, Engraulis mordax. 3 ACKNOWLEDGMENTS The Food Habits Study of Organisms of the California Current System, (Project 6–7-R), was an investigation estab- lished under contract between the U.S. -

Greenland Turbot Assessment
6HFWLRQ STOCK ASSESSMENT OF GREENLAND TURBOT James N. Ianelli, Thomas K. Wilderbuer, and Terrance M. Sample 6XPPDU\ Changes to this year’s assessment in the past year include: 1. new summary estimates of retained and discarded Greenland turbot by different target fisheries, 2. update the estimated catch levels by gear type in recent years, and 3. new length frequency and biomass data from the 1998 NMFS eastern Bering Sea shelf survey. Conditions do not appear to have changed substantively over the past several years. For example, the abundance of Greenland turbot from the eastern Bering Sea (EBS) shelf-trawl survey has found only spotty quantities with very few small fish that were common in the late 1970s and early 1980s. The majority of the catch has shifted to longline gear in recent years. The assessment model analysis was similar to last year but with a slightly higher estimated overall abundance. We attribute this to a slightly improved fit to the longline survey data trend. The target stock size (B40%, female spawning biomass) is estimated at about 139,000 tons while the projected 1999 spawning biomass is about 110,000 tons. The adjusted yield projection from F40% computations is estimated at 20,000 tons for 1999, and increase of 5,000 from last year’s ABC. Given the continued downward abundance trend and no sign of recruitment to the EBS shelf, extra caution is warranted. We therefore recommend that the ABC be set to 15,000 tons (same value as last year). As additional survey information become available and signs of recruitment (perhaps from areas other than the shelf) are apparent, then we believe that the full ABC or increases in harvest may be appropriate for this species. -

Movements of a Deep-Water Fish: Establishing Marine Fisheries Management Boundaries in Coastal Arctic Waters
University of Windsor Scholarship at UWindsor Biological Sciences Publications Department of Biological Sciences 2017 Movements of a deep-water fish: Establishing marine fisheries management boundaries in coastal Arctic waters Nigel E. Hussey University of Windsor K. J. Hedges A. N. Barkley M. A. Treble I. Peklova See next page for additional authors Follow this and additional works at: https://scholar.uwindsor.ca/biologypub Part of the Biology Commons Recommended Citation Hussey, Nigel E.; Hedges, K. J.; Barkley, A. N.; Treble, M. A.; Peklova, I.; Webber, D. M.; Ferguson, S. H.; Yurkowski, D. J.; Kessel, S. T.; Bedard, J. M.; and Fisk, A. T., "Movements of a deep-water fish: Establishing marine fisheries management boundaries in coastal Arctic waters" (2017). Ecological Applications, 27, 3, 687-704. https://scholar.uwindsor.ca/biologypub/813 This Article is brought to you for free and open access by the Department of Biological Sciences at Scholarship at UWindsor. It has been accepted for inclusion in Biological Sciences Publications by an authorized administrator of Scholarship at UWindsor. For more information, please contact [email protected]. Authors Nigel E. Hussey, K. J. Hedges, A. N. Barkley, M. A. Treble, I. Peklova, D. M. Webber, S. H. Ferguson, D. J. Yurkowski, S. T. Kessel, J. M. Bedard, and A. T. Fisk This article is available at Scholarship at UWindsor: https://scholar.uwindsor.ca/biologypub/813 INVITED FEATURE Ecological Applications, 27(3), 2017, pp. 687–704 © 2016 by the Ecological Society of America Movements of a deep- water fish: establishing marine fisheries management boundaries in coastal Arctic waters NIGEL E. -

Marine Ecology Progress Series 508:211
Vol. 508: 211–222, 2014 MARINE ECOLOGY PROGRESS SERIES Published August 4 doi: 10.3354/meps10874 Mar Ecol Prog Ser Seasonal migration, vertical activity, and winter temperature experience of Greenland halibut Reinhardtius hippoglossoides in West Greenland waters Jesper Boje1, Stefan Neuenfeldt1, Claus Reedtz Sparrevohn2, Ole Eigaard1, Jane W. Behrens1,* 1Technical University of Denmark, National Institute of Aquatic Resources, Kavalergaarden 6, 2920 Charlottenlund, Denmark 2Danish Pelagic Producers Organization, HC Andersens Boulevard 37, 1553 Copenhagen, Denmark ABSTRACT: The deep-water flatfish Greenland halibut Reinhardtius hippoglossoides (Walbaum) is common along the West Greenland coast. In the northwestern fjords, Greenland halibut is an important socio-economic resource for the Greenland community, but due to the deep and partly ice-covered environment, very little is known about its behavior and habitat characteristics. We tagged adult Greenland halibut in the waters off Ilulissat with electronic data storage tags that collected information on depth, temperature, and time. Although clear differences between individuals in migration and vertical behavior were present, we discovered a consistent seasonal migration from the relatively shallow-water Disko Bay area into the deep waters of the Ilulissat Icefjord, where the fish resided in the winter months before returning to Disko Bay. Vertical activity was pronounced at both locations, with fish covering vertical distances of up to 100 m within 15 min. During the winter months, the fish experienced temperatures between ca. 0 and 4°C, with most experiencing temperatures of 2 to 3°C. Irrespective of year and quarter of the year, the fish experienced warmer water and a broader range of temperatures when resident in Disko Bay (mean range 2.6°C) than when resident in the ice fjord (mean range 1.4°C). -

Fixed Gear Recommendations for the Cumberland Sound Greenland Halibut Fishery
Canadian Science Advisory Secretariat Central and Arctic Region Science Response 2008/011 FIXED GEAR RECOMMENDATIONS FOR THE CUMBERLAND SOUND GREENLAND HALIBUT FISHERY Context In a letter dated March 14, 2008, the Nunavut Wildlife Management Board (NWMB) requested Fisheries and Oceans Canada (DFO) Science advice on ways Greenland halibut fishing could be conducted in Cumberland Sound such that conservation concerns with non-directed by- catch of marine mammals and Greenland sharks are minimized or alleviated. On March 31, 2008, Fisheries and Aquaculture Management (FAM) submitted a request to Science for advice to address this request. Given the response was needed prior to the open-water fishing season (July 2008) and since the NWMB is the final advisory body for this request, DFO Central and Arctic Science determined that a Special Science Response Process would be used. Background The Cumberland Sound Greenland halibut (turbot) fishery began in 1986 and has been traditionally exploited during the winter months using longline gear set on the bottom through holes cut in the ice. Fishing typically takes place along a deep trench (>500 m) that extends toward Imigen Island and Drum Islands (Fig. 1). In 2005, a new management zone was established in Cumberland Sound with a Total Allowable Catch (TAC) of 500 t separate from the Northwest Atlantic Fisheries Organization (NAFO) Division 0B TAC. Catches in the winter fishery peaked in 1992 at 430 t then declined to levels below 100 t through the late 1990s and peaked again at 245 t in 2003. However, in recent years catches have declined significantly with harvests of 9 t, 70 t and 3 t for 2005, 2006 and 2007 respectively. -

Subsurface and Nighttime Behaviour of Pantropical Spotted Dolphins in Hawai′I
Color profile: Generic CMYK printer profile Composite Default screen 988 Subsurface and nighttime behaviour of pantropical spotted dolphins in Hawai′i Robin W. Baird, Allan D. Ligon, Sascha K. Hooker, and Antoinette M. Gorgone Abstract: Pantropical spotted dolphins (Stenella attenuata) are found in both pelagic waters and around oceanic is- lands. A variety of differences exist between populations in these types of areas, including average group sizes, extent of movements, and frequency of multi-species associations. Diving and nighttime behaviour of pantropical spotted dolphins were studied near the islands of Maui and Lana′i, Hawai′i, in 1999. Suction-cup-attached time–depth recorder/VHF-radio tags were deployed on six dolphins for a total of 29 h. Rates of movements of tagged dolphins were substantially lower than reported in pelagic waters. Average diving depths and durations were shallower and shorter than reported for other similar-sized odontocetes but were similar to those reported in a study of pantropical spotted dolphins in the pelagic waters of the eastern tropical Pacific. Dives (defined as >5 m deep) at night were deeper (mean = 57.0 m, SD = 23.5 m, n = 2 individuals, maximum depth 213 m) than during the day (mean = 12.8 m, SD = 2.1 m, n = 4 individuals, maximum depth 122 m), and swim velocity also increased after dark. These results, together with the series of deep dives recorded immediately after sunset, suggest that pantropical spotted dolphins around Hawai′i feed primarily at night on organisms associated with the deep-scattering layer as it rises up to the surface after dark. -

Arctic Cephalopod Distributions and Their Associated Predatorspor 146 209..227 Kathleen Gardiner & Terry A
Arctic cephalopod distributions and their associated predatorspor_146 209..227 Kathleen Gardiner & Terry A. Dick Biological Sciences, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada Keywords Abstract Arctic Ocean; Canada; cephalopods; distributions; oceanography; predators. Cephalopods are key species of the eastern Arctic marine food web, both as prey and predator. Their presence in the diets of Arctic fish, birds and mammals Correspondence illustrates their trophic importance. There has been considerable research on Terry A. Dick, Biological Sciences, University cephalopods (primarily Gonatus fabricii) from the north Atlantic and the west of Manitoba, Winnipeg, Manitoba R3T 2N2, side of Greenland, where they are considered a potential fishery and are taken Canada. E-mail: [email protected] as a by-catch. By contrast, data on the biogeography of Arctic cephalopods are doi:10.1111/j.1751-8369.2010.00146.x still incomplete. This study integrates most known locations of Arctic cepha- lopods in an attempt to locate potential areas of interest for cephalopods, and the predators that feed on them. International and national databases, museum collections, government reports, published articles and personal communica- tions were used to develop distribution maps. Species common to the Canadian Arctic include: G. fabricii, Rossia moelleri, R. palpebrosa and Bathypolypus arcticus. Cirroteuthis muelleri is abundant in the waters off Alaska, Davis Strait and Baffin Bay. Although distribution data are still incomplete, groupings of cephalopods were found in some areas that may be correlated with oceanographic variables. Understanding species distributions and their interactions within the ecosys- tem is important to the study of a warming Arctic Ocean and the selection of marine protected areas. -

Defensive Behaviors of Deep-Sea Squids: Ink Release, Body Patterning, and Arm Autotomy
Defensive Behaviors of Deep-sea Squids: Ink Release, Body Patterning, and Arm Autotomy by Stephanie Lynn Bush A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Integrative Biology in the Graduate Division of the University of California, Berkeley Committee in Charge: Professor Roy L. Caldwell, Chair Professor David R. Lindberg Professor George K. Roderick Dr. Bruce H. Robison Fall, 2009 Defensive Behaviors of Deep-sea Squids: Ink Release, Body Patterning, and Arm Autotomy © 2009 by Stephanie Lynn Bush ABSTRACT Defensive Behaviors of Deep-sea Squids: Ink Release, Body Patterning, and Arm Autotomy by Stephanie Lynn Bush Doctor of Philosophy in Integrative Biology University of California, Berkeley Professor Roy L. Caldwell, Chair The deep sea is the largest habitat on Earth and holds the majority of its’ animal biomass. Due to the limitations of observing, capturing and studying these diverse and numerous organisms, little is known about them. The majority of deep-sea species are known only from net-caught specimens, therefore behavioral ecology and functional morphology were assumed. The advent of human operated vehicles (HOVs) and remotely operated vehicles (ROVs) have allowed scientists to make one-of-a-kind observations and test hypotheses about deep-sea organismal biology. Cephalopods are large, soft-bodied molluscs whose defenses center on crypsis. Individuals can rapidly change coloration (for background matching, mimicry, and disruptive coloration), skin texture, body postures, locomotion, and release ink to avoid recognition as prey or escape when camouflage fails. Squids, octopuses, and cuttlefishes rely on these visual defenses in shallow-water environments, but deep-sea cephalopods were thought to perform only a limited number of these behaviors because of their extremely low light surroundings. -

SARSIA EGG MASSES of the SQUID GONATUS FABRICII (CEPHALOPODA, GONATIDAE) CAUGHT with PELAGIC F TRAWL OFF NORTHERN NORWAY
SHORT COMMUNICATION SARSIA EGG MASSES OF THE SQUID GONATUS FABRICII (CEPHALOPODA, GONATIDAE) CAUGHT WITH PELAGIC f TRAWL OFF NORTHERN NORWAY ƒ H e r m a n B j0 r k e , K a r s t e n H a n s e n & R o l f C. S u n d t B j 0 r k e , H e r m a n , Ka r s t e n H a n s e n & R o l f C. S u n d t 1997 08 15. Egg masses o f the squidGonatus fabricii (Cephalopoda, Gonatidae) caught with pelagic trawl off northern Norway.Sarsia - 82:149- 152. Bergen. ISSN 0036-4827. A description of egg masses from Gonatus fabricii (L ichtenstein ) caught with pelagic trawl in the Norwegian Sea is given. The eggs were kept together in a single layer between two mucous mem branes, and the pieces collected appeared to be fragments of more extensive structures tom apart by wear from the sampling gear. No embryos were observed in the eggs, and none of the eggs showed any staining for five enzyme systems analyzed by isoelectric focusing. Either the eggs were caught shortly after spawning and fertilization, or the lack of embryonic tissue reflects the fact that most of the eggs were caught in water colder than 0 °C. The developmental rate at this temperature is ex pected to be very slow. Herman Bjorke, Karsten Hansen & Rolf C. Sundt, Institute of Marine Research, P.O. Box 1870 Nordnes, N-5024 Bergen, Norway. K e y w o r d s : Cephalopoda;Gonatus fabricii', Norwegian Sea; egg masses; pelagic trawl. -
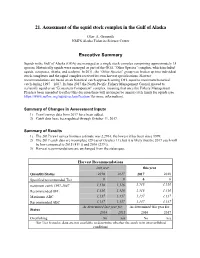
Assessment of the Squid Stock Complex in the Gulf of Alaska
21. Assessment of the squid stock complex in the Gulf of Alaska Olav A. Ormseth NMFS Alaska Fisheries Science Center Executive Summary Squids in the Gulf of Alaska (GOA) are managed as a single stock complex comprising approximately 15 species. Historically squids were managed as part of the GOA “Other Species” complex, which included squids, octopuses, sharks, and sculpins. In 2011, the “Other Species” group was broken up into individual stock complexes and the squid complex received its own harvest specifications. Harvest recommendations are based on an historical catch approach setting OFL equal to maximum historical catch during 1997 – 2007. In June 2017 the North Pacific Fishery Management Council moved to reclassify squid as an “Ecosystem Component” complex, meaning that once the Fishery Management Plan has been amended to reflect this decision there will no longer be annual catch limits for squids (see https://www.npfmc.org/squid-reclassification/ for more information). Summary of Changes in Assessment Inputs 1) Trawl survey data from 2017 have been added. 2) Catch data have been updated through October 11, 2017. Summary of Results 1) The 2017 trawl survey biomass estimate was 2,296 t, the lowest it has been since 1999. 2) The 2017 catch data are incomplete (29 t as of October 11), but it is likely that the 2017 catch will be low compared to 2015 (411 t) and 2016 (239 t). 3) Harvest recommendations are unchanged from the status quo. Harvest Recommendations last year this year Quantity/Status 2016 2017 2017 2018 Specified/recommended