Groundwater Suitability for Irrigation in Pulicat Using
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Village Panchayats in Tamil Nadu District Code District Name
List of Village Panchayats in Tamil Nadu District Code District Name Block Code Block Name Village Code Village Panchayat Name 1 Kanchipuram 1 Kanchipuram 1 Angambakkam 2 Ariaperumbakkam 3 Arpakkam 4 Asoor 5 Avalur 6 Ayyengarkulam 7 Damal 8 Elayanarvelur 9 Kalakattoor 10 Kalur 11 Kambarajapuram 12 Karuppadithattadai 13 Kavanthandalam 14 Keelambi 15 Kilar 16 Keelkadirpur 17 Keelperamanallur 18 Kolivakkam 19 Konerikuppam 20 Kuram 21 Magaral 22 Melkadirpur 23 Melottivakkam 24 Musaravakkam 25 Muthavedu 26 Muttavakkam 27 Narapakkam 28 Nathapettai 29 Olakkolapattu 30 Orikkai 31 Perumbakkam 32 Punjarasanthangal 33 Putheri 34 Sirukaveripakkam 35 Sirunaiperugal 36 Thammanur 37 Thenambakkam 38 Thimmasamudram 39 Thilruparuthikundram 40 Thirupukuzhi List of Village Panchayats in Tamil Nadu District Code District Name Block Code Block Name Village Code Village Panchayat Name 41 Valathottam 42 Vippedu 43 Vishar 2 Walajabad 1 Agaram 2 Alapakkam 3 Ariyambakkam 4 Athivakkam 5 Attuputhur 6 Aymicheri 7 Ayyampettai 8 Devariyambakkam 9 Ekanampettai 10 Enadur 11 Govindavadi 12 Illuppapattu 13 Injambakkam 14 Kaliyanoor 15 Karai 16 Karur 17 Kattavakkam 18 Keelottivakkam 19 Kithiripettai 20 Kottavakkam 21 Kunnavakkam 22 Kuthirambakkam 23 Marutham 24 Muthyalpettai 25 Nathanallur 26 Nayakkenpettai 27 Nayakkenkuppam 28 Olaiyur 29 Paduneli 30 Palaiyaseevaram 31 Paranthur 32 Podavur 33 Poosivakkam 34 Pullalur 35 Puliyambakkam 36 Purisai List of Village Panchayats in Tamil Nadu District Code District Name Block Code Block Name Village Code Village Panchayat Name 37 -

Thiruvallur District
DISTRICT DISASTER MANAGEMENT PLAN FOR 2017 TIRUVALLUR DISTRICT tmt.E.sundaravalli, I.A.S., DISTRICT COLLECTOR TIRUVALLUR DISTRICT TAMIL NADU 2 COLLECTORATE, TIRUVALLUR 3 tiruvallur district 4 DISTRICT DISASTER MANAGEMENT PLAN TIRUVALLUR DISTRICT - 2017 INDEX Sl. DETAILS No PAGE NO. 1 List of abbreviations present in the plan 5-6 2 Introduction 7-13 3 District Profile 14-21 4 Disaster Management Goals (2017-2030) 22-28 Hazard, Risk and Vulnerability analysis with sample maps & link to 5 29-68 all vulnerable maps 6 Institutional Machanism 69-74 7 Preparedness 75-78 Prevention & Mitigation Plan (2015-2030) 8 (What Major & Minor Disaster will be addressed through mitigation 79-108 measures) Response Plan - Including Incident Response System (Covering 9 109-112 Rescue, Evacuation and Relief) 10 Recovery and Reconstruction Plan 113-124 11 Mainstreaming of Disaster Management in Developmental Plans 125-147 12 Community & other Stakeholder participation 148-156 Linkages / Co-oridnation with other agencies for Disaster 13 157-165 Management 14 Budget and Other Financial allocation - Outlays of major schemes 166-169 15 Monitoring and Evaluation 170-198 Risk Communications Strategies (Telecommunication /VHF/ Media 16 199 / CDRRP etc.,) Important contact Numbers and provision for link to detailed 17 200-267 information 18 Dos and Don’ts during all possible Hazards including Heat Wave 268-278 19 Important G.Os 279-320 20 Linkages with IDRN 321 21 Specific issues on various Vulnerable Groups have been addressed 322-324 22 Mock Drill Schedules 325-336 -

The Pulicat Lake, Adjoining the Bay of Bengal Is a Shallow Water Body, Bordering the East Coast of India and Located 40 Km North of Chennai
ENVIRONMENTAL GEOCHEMISTRY OF THE PULICAT LAKE, SOUTH INDIA PURANIK GAYATRI RANGANATH JUNE-2000 ABSTRACT The Pulicat lake, adjoining the Bay of Bengal is a shallow water body, bordering the east coast of India and located 40 km north of Chennai. The lake is one of the largest salt-water lake in India that supports a wide range of socioeconomic and cultural activities. The primary aim of this study is to understand the biogeochemical processes of the Pulicat lake ecosystem in order to preserve the ecological and environmental characteristics of this fragile ecosystem. Seasonal samples of water, bed, suspended and core sediments were collected over a one-year period in three zones (southern-channel, central and northern regions) of the Pulicat lake. The lake receives fresh water discharge only during monsoon, from two seasonal rivers, the Arani discharging into the southern region of the lake (zone I) and the Kalangi discharging into the northwestern region of the lake (zone III). The Buckingham canal, running parallel to the coastline discharges sewage contaminated water and industrial effluents into the Pulicat lake. Due to the limited freshwater supply and tidal action, the mouth of the lake gets silted up and closed during the dry season. Understanding the spatial and seasonal variations in the water chemistry was one of the primary aspects of this study. The chemical composition of the surface water indicates a strong influence by seawater during the summer and diluted by river water during the monsoon season. The results obtained indicate that the dominant cations and anions of the surface water are in the order of Cl>Na>S04>K>Mg>HC03>Ca. -

Urban and Landscape Design Strategies for Flood Resilience In
QATAR UNIVERSITY COLLEGE OF ENGINEERING URBAN AND LANDSCAPE DESIGN STRATEGIES FOR FLOOD RESILIENCE IN CHENNAI CITY BY ALIFA MUNEERUDEEN A Thesis Submitted to the Faculty of the College of Engineering in Partial Fulfillment of the Requirements for the Degree of Masters of Science in Urban Planning and Design June 2017 © 2017 Alifa Muneerudeen. All Rights Reserved. COMMITTEE PAGE The members of the Committee approve the Thesis of Alifa Muneerudeen defended on 24/05/2017. Dr. Anna Grichting Solder Thesis Supervisor Qatar University Kwi-Gon Kim Examining Committee Member Seoul National University Dr. M. Salim Ferwati Examining Committee Member Qatar University Mohamed Arselene Ayari Examining Committee Member Qatar University Approved: Khalifa Al-Khalifa, Dean, College of Engineering ii ABSTRACT Muneerudeen, Alifa, Masters: June, 2017, Masters of Science in Urban Planning & Design Title: Urban and Landscape Design Strategies for Flood Resilience in Chennai City Supervisor of Thesis: Dr. Anna Grichting Solder. Chennai, the capital city of Tamil Nadu is located in the South East of India and lies at a mere 6.7m above mean sea level. Chennai is in a vulnerable location due to storm surges as well as tropical cyclones that bring about heavy rains and yearly floods. The 2004 Tsunami greatly affected the coast, and rapid urbanization, accompanied by the reduction in the natural drain capacity of the ground caused by encroachments on marshes, wetlands and other ecologically sensitive and permeable areas has contributed to repeat flood events in the city. Channelized rivers and canals contaminated through the presence of informal settlements and garbage has exasperated the situation. Natural and man-made water infrastructures that include, monsoon water harvesting and storage systems such as the Temple tanks and reservoirs have been polluted, and have fallen into disuse. -

The Institute of Road Transport Driver Training Wing, Gummidipundi
THE INSTITUTE OF ROAD TRANSPORT DRIVER TRAINING WING, GUMMIDIPUNDI LIST OF TRAINEES COMPLETED THE HVDT COURSE Roll.No:17SKGU2210 Thiru.BARATH KUMAR E S/o. Thiru.ELANCHEZHIAN D 2/829, RAILWAY STATION ST PERUMAL NAICKEN PALAYAM 1 8903739190 GUMMIDIPUNDI MELPATTAMBAKKAM PO,PANRUTTI TK CUDDALORE DIST Pincode:607104 Roll.No:17SKGU3031 Thiru.BHARATH KUMAR P S/o. Thiru.PONNURENGAM 950 44TH BLOCK 2 SATHIYAMOORTHI NAGAR 9789826462 GUMMIDIPUNDI VYASARPADI CHENNAI Pincode:600039 Roll.No:17SKGU4002 Thiru.ANANDH B S/o. Thiru.BALASUBRAMANIAN K 2/157 NATESAN NAGAR 3 3RD STREET 9445516645 GUMMIDIPUNDI IYYPANTHANGAL CHENNAI Pincode:600056 Roll.No:17SKGU4004 Thiru.BHARATHI VELU C S/o. Thiru.CHELLAN 286 VELAPAKKAM VILLAGE 4 PERIYAPALAYAM PO 9789781793 GUMMIDIPUNDI UTHUKOTTAI TK THIRUVALLUR DIST Pincode:601102 Roll.No:17SKGU4006 Thiru.ILAMPARITHI P S/o. Thiru.PARTHIBAN A 133 BLA MURUGAN TEMPLE ST 5 ELAPAKKAM VILLAGE & POST 9952053996 GUMMIDIPUNDI MADURANDAGAM TK KANCHIPURAM DT Pincode:603201 Roll.No:17SKGU4008 Thiru.ANANTH P S/o. Thiru.PANNEER SELVAM S 10/191 CANAL BANK ROAD 6 KASTHURIBAI NAGAR 9940056339 GUMMIDIPUNDI ADYAR CHENNAI Pincode:600020 Roll.No:17SKGU4010 Thiru.VIJAYAKUMAR R S/o. Thiru.RAJENDIRAN TELUGU COLONY ROAD 7 DEENADAYALAN NAGAR 9790303527 GUMMIDIPUNDI KAVARAPETTAI THIRUVALLUR DIST Pincode:601206 Roll.No:17SKGU4011 Thiru.ULIS GRANT P S/o. Thiru.PANNEER G 68 THAYUMAN CHETTY STREET 8 PONNERI 9791745741 GUMMIDIPUNDI THIRUVALLUR THIRUVALLUR DIST Pincode:601204 Roll.No:17SKGU4012 Thiru.BALAMURUGAN S S/o. Thiru.SUNDARRAJAN N 23A,EGAMBARAPURAM ST 9 BIG KANCHEEPURAM 9698307081 GUMMIDIPUNDI KANCHEEPURAM DIST Pincode:631502 Roll.No:17SKGU4014 Thiru.SARANRAJ M S/o. Thiru.MUNUSAMY K 5 VOC STREET 10 DR. -

Marine Infrastructure Developer Private Limited (MIDPL)
Marine Infrastructure Developer Private Limited (MIDPL) Executive Summary PROPOSED REVISED MASTER PLAN DEVELOPMENT OF KATTUPALLI PORT December 2020 PREPARED BY C1161303 NABET ACCREDITED Certificate No: NABET/EIA/2023/RA 0175 RP003, Rev. A L&T Infrastructure Engineering Ltd. Client: Marine Infrastructure Developer Private Limited (MIDPL) Project: Proposed Revised Master Plan Project No.: Development of Kattupalli Port C1161303 Title: Document No.: Rev.: Executive Summary RP003 A This document is the property of L&T Infrastructure Engineering Ltd. and File path: must not be passed on to any person or body not authorised by us to receive it l:\ports\2016\c1161303 - ceia kattupalli port nor be copied or otherwise made use of either in full or in part by such person or expansion\outputs\reports\rp003-executive summary\13.12.2020\executive body without our prior permission in writing. summary-14.12.2020.docx Notes: 1. Revision Details: SAP TKS A 13.12.2020 Second Submission SNV BRT S SNV SAP TKS 0 26.10.2020 First Submission Sd/- Sd/- Sd/- IRR BRT S Init. Sign. Init. Sign. Init. Sign. Rev. Date Details Prepared Checked Approved Table of Contents Proposed Revised Master Plan Development of Kattupalli Port C1161303 Executive Summary RP003 rev. A TABLE OF CONTENTS 1 Introduction .....................................................................................................................................1 2 Project Site ......................................................................................................................................1 -

Need of Coastal Resource Management in Pulicat Lake–Challenges Ahead
322 Indian Journal of Science and Technology Vol. 4 issue 3 (March 2011) ISSN: 0974- 6846 Need of coastal resource management in Pulicat Lake–challenges ahead N. Thirunavukkarasu, S. Gokulakrishnan, P. V. R. Premjothi and R. Moses Inbaraj Dept. of Marine Studies and Coastal Resource Management, Madras Christian College, Tambaram, Chennai–59, India [email protected] Abstract Coastal zones are currently experiencing intense and sustained environmental pressures from a range of driving forces. Responsible agencies around the globe are seeking ways of better managing the causes and consequences of the environmental change process in coastal areas. The demands on the environment are raising serious concerns from environmentalists, stakeholders, coastal communities and researchers. Pulicat Lake is the second largest lagoon in India has rich natural but at the same time very fragile ecosystem. This lagoon provides nursery and breeding grounds for many species of marine fauna and supports commercial fishing too. This article discusses the current status of the Pulicat Lake biodiversity, the ecological crisis faced by the lake due to lake-mouth closure issues, siltation, shrinkage of the lake, pollution, over fishing, degradation and destruction of natural habitats in the environment. Further it focus on fishermen community socio-economic perspectives of their development towards livelihoods, social organization, literacy, fishing pattern, marketing outlet, income and involvement by NGO’s etc. The healthier lake needs integrated policy approaches, which involve scientific disciplines to address the complexity of the interaction between the social and natural systems in the coastal and marine environment. Keywords: Pulicat Lake, fragile ecosystem, siltation, literacy, fishing pattern. Introduction there is a need to conserve the ecosystem with potential Pulicat Lake is the second largest brackish water lake, biodiversity resources as well as fishermen community lying partly in Tamil Nadu and Andhra Pradesh. -

Battlefield Through Bird Sanctuary - the Pulicat Lake
Battlefield through Bird Sanctuary - the Pulicat Lake Vaithiyanathan Kannan Research Scholar Research Department of Zoology and Wildlife Biology, A.V.C. College (Autonomous), Mayiladuthurai, Mannampandal 605 309, India Email: [email protected] Pulicat Lake was most popular busy town right from the 6 th to 13 th century A.D. The existence of Pulicat can be traced back from the Cholas. Pulicat has evolved into an urban centre and the successive stages of evolution of pulicat can be classified in to Chola period (until 13 th century A.D.), Nayak period (14 th to 16 th century A.D), Colonial period (17 th century to 1947 A.D.) and Post Independence period. Chola and Nayak Period Pulicat was under the Imperial Cholas who first built temple at Thiruppalaivanam near Pulicat. The Cholas divided their territories into kottams (presently called district). Pulicat was referred to as Payyar Kottam, Puliyur Kottam and Pular Kottam. Veeran Thirubuvana Chakravarthy ruled the Puliyur Kottam. Latter on during the Nayak period the Vijayanagar kingdom was founded in 1336 A.D., which became a dominant force in Tamil Nadu where Viswanath Nayak was ruling Madurai in 1529. Under the Nayaks the Vijayanagar kingdom was frequently waged war against the native Tamil rulers and brought the entire land under their control. Due to the conflicts within the Nayak groups which enabled the sultans of Bijapur and Golconda to penetrate the Tamil Country. The battle of Thalaikotta (1565) enabled the sultans to capture some regions of the Vijayanagar kingdom and thus pulicat came under their rule. In 1572, Thirumalai Nayak, who had been vassal of the sultan, divided his kingdom into three parts, one of which was given to Venkatapathi who ruled it from Chandragiri as his capital. -

7D. Fisherfolk in TN.P65
7d. FISHER FOLK IN TAMILNADU The fisheries sector, both marine and inland, plays an important role in Tamilnadu’s economy. And fish resources are of utmost importance for food security. Tamilnadu has a long coastline of about 1000 kms, accounting for about 17% of the Indian coastline. Of this, the Coramandal coast covers about 350 kms, the Palk Bay about 270 kms, the Gulf of Mannar about 320 kms and the west coast about 60 kms. The continental shelf of Tamilnadu has got an area of 41,412 sq.kms. The exclusive economic zone of Tamilnadu is 1.9 lakhs sq.kms. The Tamilnadu coast has nearly 26 big and small urban centres and 556 marine fishing villages located along the 12 maritime districts. Marine fish landing takes place in 362 centres. The major landing centres in Tamilnadu coast are Ennore, Chennai, Cuddalore, Port Novo, Nagappattinam, Athirampattinam, Rameswaram, Pamban, Thoothukudi, Kanyakumari and Colachel. Besides the coast, there are 5 major rivers, 51 reservoirs and innumerable lakes, ponds and tanks in Tamilnadu, providing much scope for inland fishing. The State has a potential of 3.74 lakh hectares of culturable water-spread for developing inland fisheries. Clear statistics with regard to even the number of fisherfolk population in Tamilnadu are not available. As per the last Census of marine fisherfolk in 1986, there were 4.64 lakhs marine fisherfolk, out of whom, 1.01 lakhs were actively engaged in fishing. In 1993, as per the statistics of the Department of Fisheries, the fisherfolk population of Tamilnadu was calculated at 5.15 lakhs of whom about 1.81 lakh fishermen were actively involved in fishing. -
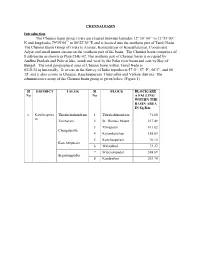
Introduction the Chennai Basin Group Rivers Are Situated Between
CHENNAI BASIN Introduction The Chennai basin group rivers are situated between latitudes 12o 30’ 00’’ to 13 o35’00’’ N and longitudes 79o15’00’’ to 80o22’30’’E and is located into the northern part of Tamil Nadu. The Chennai Basin Group of rivers is Araniar, Korattalaiyar or Kosasthalaiyar, Cooum and Adyar and small minor stream on the southern part of the basin. The Chennai basin comprises of 8 sub basins as shown in Plate:CHE-02. The northern part of Chennai basin is occupied by Andhra Pradesh and Pulicat lake, south and west by the Palar river basin and east by Bay of Bengal. The total geographical area of Chennai basin within Tamil Nadu is 6118.34 sq km totally. It covers in the Survey of India toposheets 57‘O’, 57 ‘P’, 66’C’, and 66 ‘D’ and it also covers in Chennai, Kancheepuram, Thiruvallur and Vellore districts. The administrative setup of the Chennai basin group is given below (Figure 1) Sl DISTRICT TALUK Sl BLOCK BLOCK/ARE No No A FALLING WITHIN THE BASIN AREA IN Sq Km 1. Kancheepura Thirukalunkundram 1 Thirukalukundram 71.60 m Tambaram 2 St. Thomas Mount 217.49 3 Thiruporur 411.82 Chengalpattu 4 Kattankulathur 158.83 5 Kancheepuram 10.14 Kancheepuram 6 Walajabad 73.27 7 Sriperumpudur 248.69 Sriperumpudur 8 Kundrathur 203.70 BLOCK/ARE A FALLING Sl Sl DISTRICT TALUK BLOCK WITHIN THE No No BASIN AREA IN Sq Km Gummidipoond Gummidipoondi 9 420.51 i 10 Minjur 478.69 Ponneri 11 Cholavaram 193.85 12 Puzhal 127.10 Ambattur 13 Villivakkam 210.61 Poonamallee 14 Poonamallee 178.33 2. -

Indian Textiles in the Indian Ocean Trade in the Early Modern Period
Indian Textiles in the Indian Ocean Trade In the Early Modern Period Om Prakash* The Indian Ocean is by far the oldest of the seas in history, in terms of it being used and traversed by humans. Intense commercial activity has been carried out in the Ocean at least over the last two millennia. Networks of trade covering different segments of the Ocean have a history of remarkable resilience without being resistant to innovation. While all kinds of commodities, including precious metals, have figured in the Indian Ocean trade, textiles both for mass as well as elite consumption have always had a very special place, both qualitatively, as well as quantitatively, in this trade. In addition to being used for wearing apparel purposes and as furnishings, textiles have also had an important function to perform in the domain of rituals, exchange of gifts, identity formation and so on. In the domain of economics, textiles often served as currency and as medium of exchange. Being probably the largest, and perhaps the most cost-competitive, producer of textiles of all varieties for centuries, India has been at the centre of Indian Ocean trade in textiles for a long period of time. Indian textiles have figured prominently both in the trade with west Asia and the Mediterranean via the Arabia Sea as well as with mainland and island southeast Asia via the Bay of Bengal. As for the first of these regions, the first century A.D. Periplus Maris * Delhi School of Economics, University of Delhi, Delhi-110007 INDIA. Email: [email protected] . -
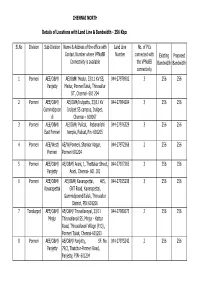
CHENNAI NORTH Sl.No Division Sub-Division Name & Address Of
CHENNAI NORTH Details of Locations with Land Line & Bandwidth - 256 Kbps Sl.No Division Sub-Division Name & Address of the office with Land Line No. of PCs Contact Number where VPNoBB Number connected with Existing Proposed Connectivity is available the VPNoBB Bandwidth Bandwidth connectivity 1 Ponneri AEE/O&M/ AE/O&M/ Medur, 33/11 KV SS, 044-27978902 3 256 256 Panjetty Medur, PonneriTaluk, Thiruvallur DT, Chennai- 601 204 2 Ponneri AEE/O&M/ AE/O&M/Irulipattu, 33/11 KV 044-27984204 3 256 256 Gummidipoon Irulipet SS campus, Irulipet, di Chennai – 600067 3 Ponneri AEE/O&M/ AE/O&M/ Pulicat, Pabanarishi 044-27976329 3 256 256 East Ponneri temple, Pulicat, Pin -601205 4 Ponneri AEE/West/ AE/W/Ponneri, Shankar Nagar, 044-27972368 2 256 256 Ponneri Ponneri 601204 5 Ponneri AEE/O&M/ AE/O&M/ Arani, 1, Thottakar Street, 044-27927265 3 256 256 Panjetty Arani, Chennai- 601 101 6 Ponneri AEE/O&M/ AE/O&M/ Kavarapettai, 465, 044-27925238 3 256 256 Kavarapettai GNT Road, Kavarapettai, GummidipoondiTaluk, Thiruvallur District, PIN 601206 7 Tondiarpet AEE/O&M/ AE/O&M/ Tiruvellavoyal, 33/11 044-27980675 2 256 256 Minjur Thiruvallavoil SS, Minjur - Kattur Road, Thiruvallavoil Village (P.O) , Ponneri Taluk, Chennai-601203 8 Ponneri AEE/O&M/ AE/O&M/ Panjetty, SF. No. 044-27975242 2 256 256 Panjetty 79/2, Thatchur-Ponneri Road, Panjetty, PIN- 601204 Details of Locations with Land Line & Bandwidth - 512 Kbps Sl.No Division Sub-Division Name & Address of the office with Land Line No. of PCs Contact Number where VPNoBB Number connected with Existing Proposed Connectivity is available the VPNoBB Bandwidth Bandwidth connectivity 1 T.Nagar Teynampet DMS SS, DMS Complex, Anna Salai, 044-24332950 3 512 512 Chennai – 6 2 T.Nagar Saidapet Thodunter Nagar SS, No.22, 044-24322211 3 512 512 Thodunter Nagar, Saidapet, CH- 15 3 T.Nagar Saidapet MHU SS, No.1 Link Road, MHU 044-24363191 1 512 512 Compound, CIT Nagar, Ch-35.