B&T Cell Associated Lymphoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

IL-7 Receptor Blockade Blunts Antigen-Specific Memory T Cell
ARTICLE DOI: 10.1038/s41467-018-06804-y OPEN IL-7 receptor blockade blunts antigen-specific memory T cell responses and chronic inflammation in primates Lyssia Belarif1,2, Caroline Mary1,2, Lola Jacquemont1, Hoa Le Mai1, Richard Danger1, Jeremy Hervouet1, David Minault1, Virginie Thepenier1,2, Veronique Nerrière-Daguin1, Elisabeth Nguyen1, Sabrina Pengam1,2, Eric Largy3,4, Arnaud Delobel3, Bernard Martinet1, Stéphanie Le Bas-Bernardet1,5, Sophie Brouard1,5, Jean-Paul Soulillou1, Nicolas Degauque 1,5, Gilles Blancho1,5, Bernard Vanhove1,2 & Nicolas Poirier1,2 1234567890():,; Targeting the expansion of pathogenic memory immune cells is a promising therapeutic strategy to prevent chronic autoimmune attacks. Here we investigate the therapeutic efficacy and mechanism of new anti-human IL-7Rα monoclonal antibodies (mAb) in non-human primates and show that, depending on the target epitope, a single injection of antagonistic anti-IL-7Rα mAbs induces a long-term control of skin inflammation despite repeated antigen challenges in presensitized monkeys. No modification in T cell numbers, phenotype, function or metabolism is observed in the peripheral blood or in response to polyclonal stimulation ex vivo. However, long-term in vivo hyporesponsiveness is associated with a significant decrease in the frequency of antigen-specific T cells producing IFN-γ upon antigen resti- mulation ex vivo. These findings indicate that chronic antigen-specific memory T cell responses can be controlled by anti-IL-7Rα mAbs, promoting and maintaining remission in T- cell mediated chronic inflammatory diseases. 1 Centre de Recherche en Transplantation et Immunologie (CRTI) UMR1064, INSERM, Université de Nantes, Nantes 44093, France. 2 OSE Immunotherapeutics, Nantes 44200, France. -

PAX5 Expression in Acute Leukemias: Higher B-Lineage Specificity Than Cd79a and Selective Association with T(8;21)-Acute Myelogenous Leukemia
[CANCER RESEARCH 64, 7399–7404, October 15, 2004] PAX5 Expression in Acute Leukemias: Higher B-Lineage Specificity Than CD79a and Selective Association with t(8;21)-Acute Myelogenous Leukemia Enrico Tiacci,1 Stefano Pileri,2 Annette Orleth,1 Roberta Pacini,1 Alessia Tabarrini,1 Federica Frenguelli,1 Arcangelo Liso,3 Daniela Diverio,4 Francesco Lo-Coco,5 and Brunangelo Falini1 1Institutes of Hematology and Internal Medicine, University of Perugia, Perugia, Italy; 2Unit of Hematopathology, University of Bologne, Bologne, Italy; 3Section of Hematology, University of Foggia, Foggia, Italy; 4Department of Cellular Biotechnologies and Hematology, University La Sapienza of Rome, Rome, Italy; and 5Department of Biopathology, University Tor Vergata of Rome, Rome, Italy ABSTRACT (13, 16). PAX5 expression also occurs in the adult testis and in the mesencephalon and spinal cord during embryogenesis (17), suggesting an The transcription factor PAX5 plays a key role in the commitment of important role in the development of these tissues. hematopoietic precursors to the B-cell lineage, but its expression in acute Rearrangement of the PAX5 gene through reciprocal chromosomal leukemias has not been thoroughly investigated. Hereby, we analyzed routine biopsies from 360 acute leukemias of lymphoid (ALLs) and mye- translocations has been described in different types of B-cell malig- loid (AMLs) origin with a specific anti-PAX5 monoclonal antibody. Blasts nancies (18–23), and, more recently, PAX5 has also been shown to be from 150 B-cell ALLs showed strong PAX5 nuclear expression, paralleling targeted by aberrant hypermutation in Ͼ50% of diffuse large B-cell that of CD79a in the cytoplasm. Conversely, PAX5 was not detected in 50 lymphomas (24). -

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -
Bispecific CAR-T Cells Targeting Both CD19 and CD22 for Therapy Of
Dai et al. Journal of Hematology & Oncology (2020) 13:30 https://doi.org/10.1186/s13045-020-00856-8 RAPID COMMUNICATION Open Access Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia Hanren Dai1,2,3†, Zhiqiang Wu1†, Hejin Jia2†, Chuan Tong1, Yelei Guo1, Dongdong Ti1, Xiao Han1, Yang Liu4, Wenying Zhang2, Chunmeng Wang2, Yajing Zhang2, Meixia Chen2, Qingming Yang2, Yao Wang1* and Weidong Han1,2* Abstract Background: Despite the impressive complete remission (CR) induced by CD19 CAR-T cell therapy in B-ALL, the high rate of complete responses is sometimes limited by the emergence of CD19-negative leukemia. Bispecific CAR-modified T cells targeting both CD19 and CD22 may overcome the limitation of CD19-negative relapse. Methods: We here report the design of a bispecific CAR simultaneous targeting of CD19 and CD22. We performed a phase 1 trial of bispecific CAR T cell therapy in patients with relapsed/refractory precursor B-ALL at a dose that ranged from 1.7 × 106 to 3 × 106 CAR T cells per kilogram of body weight. Results: We demonstrate bispecific CD19/CD22 CAR T cells could trigger robust cytolytic activity against target cells. MRD-negative CR was achieved in 6 out of 6 enrolled patients. Autologous CD19/CD22 CAR T cells proliferated in vivo and were detected in the blood, bone marrow, and cerebrospinal fluid. No neurotoxicity occurred in any of the 6 patients treated. Of note, one patient had a relapse with blast cells that no longer expressed CD19 and exhibited diminished CD22 site density approximately 5 months after treatment. -

CD81 Is Required for CD19-Complex Formation and Terminal Human B
Supplemental Table 1. Primer sequences for PCR amplification and sequencing of CD81 coding regions from genomic DNA. Exon Forward primer Forward primer sequence Reverse primer Reverse primer sequence 1 CD81exon1F GGGGCGGGGCCTATGGAG CD81exon1R GGACCTGCCCAACGTGGA 2 CD81exon2F TGTGGGGTGGGCGCACTC CD81exon2R CACGCCATGCCCGACTGT 3 CD81exon3F ATCCCTGGCAGTCAGCAACC CD81exon3R TCCGCCCTGAGCACCAGC 4 CD81exon4F GTCAGGTCGTGGGCTGGT CD81exon4R CTGGAGATCCTCCTGGCAAGT 5 CD81exon5F TCTGGGGTCTAGCCTCGAAGC CD81exon5R CTGGGCGTAGGCAGGATT 6 CD81exon6F GGCCCCTGGATGCATTCT CD81exon6R AGTGTGGTCGCTCCCTGTGG 7+8 CD81exon7+8F CTGCGTGACAACGGGAAG CD81exon7+8R TATACACAGGCGGTGATGG Supplemental Table 2. Primer sequences for PCR amplification and sequencing of CD81 and CD225 transcripts. Gene Forward primer Forward primer sequence Reverse primer Reverse primer sequence CD81 CD81_mRNA_F1 GACCCCACCGCGCATCCT CD81_mRNA_R1 GGATGGCCCCGTAGCAGC CD81_mRNA_F2 CGCCCAACACCTTCTATGTA CD81_mRNA_R2 TGCCCGAGGGACACAAAT CD81_mRNA_F3 TTCCACGAGACGCTTGACTGCT CD81_mRNA_R3 AGGCCCGTCTCCACTCAT IFITM1 IFITM1_mRNA_F1 TCATTGGTCCCTGGCTAATTCAC IFITM1_mRNA_R1 GGTCACGTCGCCAACCAT IFITM1_mRNA_F2 ACAGCGAGACCTCCGTGC IFITM1_mRNA_R2 TCTAGGGGCAGGACCAAG Supplemental Table 3. PCR primers and TaqMan probes for CD81 transcript level quantification. Target Forward primer Forward primer sequence Reverse primer Reverse primer sequence TaqMan probe TaqMan probe Sequence total CD81 CD81_RQ_F CGCCAAGGCTGTGGTGAA CD81_RQ_R AGAGGTTGCTGATGATGTTGCTG T-CD81 ACTGACTGCTTTGACCACCTCAGTGCTCA wild type CD81 CD81_RQ_F CGCCAAGGCTGTGGTGAA -

CD19 Chimeric Antigen Receptor-Exosome Targets CD19 Positive B-Lineage Acute Lymphocytic Leukemia and Induces Cytotoxicity
cancers Article CD19 Chimeric Antigen Receptor-Exosome Targets CD19 Positive B-lineage Acute Lymphocytic Leukemia and Induces Cytotoxicity Shabirul Haque 1,2,* and Sarah R. Vaiselbuh 1,2,3 1 Feinstein Institute for Medical Research, Northwell Health, 350 Community Drive, Manhasset, NY 11030, USA; [email protected] 2 Department of Pediatrics, Staten Island University Hospital, Northwell Health, 475 Seaview Ave, Staten Island, NY 10305, USA 3 Monsey Health Center, 40 Robert Pitt Drive, Monsey, NY 10952, USA * Correspondence: [email protected] Simple Summary: Our research describes our designer exosomes express CD19 Chimeric Antigen Receptor (Exo-CD19 CAR). This novel Exo-CD19 CAR is cytotoxic for CD19-positive leukemia B-cells without interfering with cytotoxicity in CD19-negative cells. This innovation can be translated into broader clinical applications as CD19 CAR exosome-based nano-immunotherapy for B-cell leukemia instead of whole CD19 CAR T-cell immunotherapy. Abstract: CAR-T cell therapy is not without some clinical adverse effects, namely cytokine storms, due to a massive release of cytokines when CAR-T cells multiply in the body. Our goal was to develop exosomes expressing CD19 CAR to treat CD19-positive B-cell malignancies, instead of using whole CD19 CAR-T cells, thereby reducing the clinical risk of uncontrolled cytokine storms. Exosomes are Citation: Haque, S.; Vaiselbuh, S.R. extracellular nanovesicles (30–150 nm), composed of lipids, proteins, and nucleic acids, that carry the CD19 Chimeric Antigen fingerprint of their parent cells. Exosomes are a preferred delivery system in nano-immunotherapy. Receptor-Exosome Targets CD19 Here, HEK293T parent cells were transduced with CD19 CAR plasmids and cellular CD19 CAR Positive B-lineage Acute Lymphocytic expression was confirmed. -

ORIGINAL ARTICLE Flow Cytometric Protein Expression Profiling As a Systematic Approach for Developing Disease-Specific Assays
Leukemia (2006) 20, 2102–2110 & 2006 Nature Publishing Group All rights reserved 0887-6924/06 $30.00 www.nature.com/leu ORIGINAL ARTICLE Flow cytometric protein expression profiling as a systematic approach for developing disease-specific assays: identification of a chronic lymphocytic leukaemia-specific assay for use in rituximab-containing regimens AC Rawstron, R de Tute, AS Jack and P Hillmen Haematological Malignancy Diagnostic Service (HMDS), Leeds Teaching Hospitals, Leeds, UK Depletion of disease below the levels detected by sensitive sustained remissions only occur in patients achieving an MRD- minimal residual disease (MRD) assays is associated with negative complete response.12 Therefore MRD is increasingly prolonged survival in chronic lymphocytic leukaemia (CLL). being used as an end point for therapeutic trials, and several Flow cytometric MRD assays are now sufficiently sensitive and rapid to guide the duration of therapy in CLL, but generally rely studies are now using the assessment of MRD to define the on assessment of CD20 expression, which cannot be accurately duration of therapy. measured during and after therapeutic approaches containing Approaches using allele-specific oligonucleotide polymerase rituximab. The aim of this study was to use analytical software chain reaction (ASO-PCR) to the immunoglobulin gene of the developed for microarray analysis to provide a systematic B-CLL cell are generally accepted to show the highest sensitivity approach for MRD flow assay development. Samples from CLL for MRD detection. However, more recent four-colour ap- patients (n ¼ 49), normal controls (n ¼ 21) and other B-lympho- proaches show sensitivities nearing that of ASO-PCR6,11,13 with proliferative disorders (n ¼ 12) were assessed with a panel of 66 antibodies. -

New Advances in Leukaemia Immunotherapy by the Use of Chimeric Artificial Antigen Receptors
Biagi et al. Italian Journal of Pediatrics 2011, 37:46 http://www.ijponline.net/content/37/1/46 ITALIAN JOURNAL OF PEDIATRICS REVIEW Open Access New advances in leukaemia immunotherapy by the use of Chimeric Artificial Antigen Receptors (CARs): state of the art and perspectives for the near future Ettore Biagi*, Virna Marin, Greta Maria Paola Giordano Attianese, Irene Pizzitola, Sarah Tettamanti, Elisabetta Cribioli and Andrea Biondi Abstract Leukaemia immunotherapy represents a fascinating and promising field of translational research, particularly as an integrative approach of bone marrow transplantation. Adoptive immunotherapy by the use of donor-derived expanded leukaemia-specific T cells has showed some kind of clinical response, but the major advance is nowadays represented by gene manipulation of donor immune cells, so that they acquire strict specificity towards the tumour target and potent lytic activity, followed by significant proliferation, increased survival and possibly anti- tumour memory state. This is achieved by gene insertion of Chimeric T-cell Antigen Receptors (CARs), which are artificial molecules containing antibody-derived fragments (to bind the specific target), joined with potent signalling T-Cell Receptor (TCR)-derived domains that activate the manipulated cells. This review will discuss the main application of this approach particularly focusing on the paediatric setting, raising advantages and disadvantages and discussing relevant perspectives of use in the nearest future. Keywords: Leukaemia immunotherapy, -
![Cd1a [O10] Concentrated and Prediluted Monoclonal Antibody 901-3158-061719](https://docslib.b-cdn.net/cover/1446/cd1a-o10-concentrated-and-prediluted-monoclonal-antibody-901-3158-061719-341446.webp)
Cd1a [O10] Concentrated and Prediluted Monoclonal Antibody 901-3158-061719
CD1a [O10] Concentrated and Prediluted Monoclonal Antibody 901-3158-061719 Catalog Number: ACI 3158 A, B API 3158 AA VLTM 3158 G20 Description: 0.1, 0.5 mL conc. 6.0 mL, RTU 20 mL, RTU Dilution: 1:100 Ready-to-use Ready-to-use Diluent: Van Gogh Yellow N/A N/A Intended Use: Protocol Recommendations (VALENT® Automated Slide For In Vitro Diagnostic Use Staining Platform) Cont’d: CD1a [O10] is a mouse monoclonal antibody that is intended for Protein Block (Optional): Incubate for 10-20 minutes at RT with Val laboratory use in the qualitative identification of CD1a protein by Background Block. immunohistochemistry (IHC) in formalin-fixed paraffin-embedded Primary Antibody: Incubate for 30 minutes. (FFPE) human tissues. The clinical interpretation of any staining or its Secondary: Incubate for 10 minutes with Val Mouse Secondary. absence should be complemented by morphological studies using proper Linker: Incubate for 10 minutes with Val Universal Linker. controls and should be evaluated within the context of the patient’s Polymer: Incubate for 10 minutes with Val Universal Polymer. clinical history and other diagnostic tests by a qualified pathologist. Chromogen: Incubate for 5 minutes with Val DAB. Summary and Explanation: Counterstain: Counterstain for 5 minutes with Val Hematoxylin. CD1a is a protein of 43 to 49 kDa and is expressed on dendritic cells and cortical thymocytes (1,2). CD1a [O10] staining has been shown to be Protocol Recommendations (intelliPATH FLX® and manual use): useful in the differentiation of Langerhans cells from interdigitating cells. Peroxide Block: Block for 5 minutes with Peroxidazed 1. It has also proved useful for phenotyping Langerhans cell histiocytosis Pretreatment: Perform heat retrieval using Diva or Reveal Decloaker. -

Single-Cell RNA Sequencing Demonstrates the Molecular and Cellular Reprogramming of Metastatic Lung Adenocarcinoma
ARTICLE https://doi.org/10.1038/s41467-020-16164-1 OPEN Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma Nayoung Kim 1,2,3,13, Hong Kwan Kim4,13, Kyungjong Lee 5,13, Yourae Hong 1,6, Jong Ho Cho4, Jung Won Choi7, Jung-Il Lee7, Yeon-Lim Suh8,BoMiKu9, Hye Hyeon Eum 1,2,3, Soyean Choi 1, Yoon-La Choi6,10,11, Je-Gun Joung1, Woong-Yang Park 1,2,6, Hyun Ae Jung12, Jong-Mu Sun12, Se-Hoon Lee12, ✉ ✉ Jin Seok Ahn12, Keunchil Park12, Myung-Ju Ahn 12 & Hae-Ock Lee 1,2,3,6 1234567890():,; Advanced metastatic cancer poses utmost clinical challenges and may present molecular and cellular features distinct from an early-stage cancer. Herein, we present single-cell tran- scriptome profiling of metastatic lung adenocarcinoma, the most prevalent histological lung cancer type diagnosed at stage IV in over 40% of all cases. From 208,506 cells populating the normal tissues or early to metastatic stage cancer in 44 patients, we identify a cancer cell subtype deviating from the normal differentiation trajectory and dominating the metastatic stage. In all stages, the stromal and immune cell dynamics reveal ontological and functional changes that create a pro-tumoral and immunosuppressive microenvironment. Normal resident myeloid cell populations are gradually replaced with monocyte-derived macrophages and dendritic cells, along with T-cell exhaustion. This extensive single-cell analysis enhances our understanding of molecular and cellular dynamics in metastatic lung cancer and reveals potential diagnostic and therapeutic targets in cancer-microenvironment interactions. 1 Samsung Genome Institute, Samsung Medical Center, Seoul 06351, Korea. -
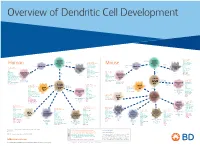
View Dendritic Cell Development Poster
Overview of Dendritic Cell Development Lineage–, CD45+, Common CD117 (c-kit) Common MHCII+, CD11c+ – + CD207 (Langerin) Myeloid CD117 (c-kit) Lineage , CD45 , Myeloid Progenitor MHCII (HLA-DR)+, CD11c+ Progenitor CD324 (E-Cadherin) Human Mouse CD326 (EpCAM) CD207 (Langerin) TGFb1 Cells CD11b, CD115 Cells CD14 Monocyte CD324 (E-Cadherin) Monocyte M-CSF CD11b – + Ly6C Langerhans CD24 Lineage , CD45 , M-CSF CD326 (EpCAM) MHCII (HLA-DR)+, CD11c+ Langerhans CD11blo Zbtb46– Cells CD172a (Sirp-α) CD16 CD1ahi, CD1c CD205 (DEC-205) Cells CSF F4/80 CD64 CD172a (Sirp-α) Lineage–, CD45+, FLT3L TLR3, TLR11 CD1a, CD1c Inflammatory CD369 (Dectin-1/CLEC7A) MHCII+, CD11c+ +/– CSF IL-15 CD8–, CD14– CD11b, CD14 CD371 (CLEC12A) CD64 Monocyte- FLT3L Inflammatory CD370 (Clec9a)– CD172a (Sirp-α) IL-15 CLEC6A CD11b derived lo Monocyte- CD206, CD209 (DC-SIGN) TLR1, TLR2, TLR3 , TLR6 CD209a (DC-SIGN) CD367 (DCIR/CLEC4A) DCs CD14– CD272 (BTLA)lo derived CD369 (Dectin-1/CLEC7A) DCs Common Ly-6C – + CD371 (CLEC12A) CD117 (c-kit) Lineage , CD45 , IL-1β, IL-6, IL-10, TLR1-6, TLR7-8, TLR10 Dendritic + lo CLEC6A – – CD135/FLT3 MHCII , CD11c IL-12, IL-23, TNF CD8a , CD14 IL-1β, IL-6 IL-10, Precursor TLR3lo, TLR4, TLR7, TLR8 CD45R (B220) IL-12, IL-23, TNF Plasmacytoid CD207 (Langerin)– Cells CD317 (BST-2) Common Lineage–, CD45+, FLT3L DCs Lineage–, CD45+, + Ly6C + lo/– CD207 IFN Type I + + Dendritic CD135/FLT3 MHCII (HLA-DR) , CD11c Lineage–, CD45+, IRF7, IRF8, BATF3hi Siglec-H MHCII (HLA-DR) , CD11c hi – + CD123 + + Dermal SpiB, Zbtb46 CD1a, CD64 CD1a Precursor CD117 (c-kit) -

Immunohistochemical Expression of CD23 and CD40 May Identify Prognostically Favorable Subgroups of Diffuse Large B-Cell Lymphoma: a Nordic Lymphoma Group Study1
722 Vol. 9, 722–728, February 2003 Clinical Cancer Research Immunohistochemical Expression of CD23 and CD40 May Identify Prognostically Favorable Subgroups of Diffuse Large B-cell Lymphoma: A Nordic Lymphoma Group Study1 Johan Linderoth,2 Mats Jerkeman, to a germinal center origin or attributable to increased Eva Cavallin-Ståhl, Stein Kvaløy, and apoptosis via induction of bax and/or enhanced T-cell inter- Emina Torlakovic action, resulting in improved autologous tumor response. Confirmatory studies are necessary. Department of Oncology, Lund University Hospital, 221 85 Lund, Sweden [J. L., M. J., E. C-S.]; Department of Oncology, the Norwegian Radium Hospital, Oslo, Norway [S. K.]; and Department INTRODUCTION of Pathology, The Norwegian Radium Hospital, Oslo, Norway [E. T.] DLBCL3 is the most frequent lymphoma subtype and en- compasses the majority, ϳ60–70%, of the aggressive lympho- mas. Biologically and clinically, it shows considerable hetero- ABSTRACT geneity. Eventually, 30–40% of the patients with advanced Purpose: In search for subgroups of diffuse large B-cell stage DLBCL who are treated with current therapies will be lymphoma (DLBCL) with different histogenetic origin and long-time survivors, whereas the rest will succumb to the dis- prognosis, as has been described by gene expression profil- ease. There is an obvious need for prognostic information to ing, we examined tumor specimens from 125 patients with design a more risk-adapted primary therapy. The IPI is one DLBCL, uniformly treated by either cyclophosphamide- available, validated tool, based on clinical features (age, stage, Adriamycin-vincristine-prednisone or methotrexate, doxo- level of lactate dehydrogenase, performance status, and number rubicin, cyclophosphamide, vincristine, prednisone, and of extranodal sites; Ref.