Investigating Changes in Tactile and Proprioceptive
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Somatosensory Processes Subserving Perception and Action
BEHAVIORAL AND BRAIN SCIENCES (2007) 30, 189–239 Printed in the United States of America DOI: 10.1017/S0140525X07001392 Somatosensory processes subserving perception and action H. Chris Dijkerman Department of Experimental Psychology, Helmholtz Research Institute, Utrecht University, 3584 CS Utrecht, The Netherlands [email protected] Edward H. F. de Haan Department of Experimental Psychology, Helmholtz Research Institute, Utrecht University, 3584 CS Utrecht, The Netherlands [email protected] Abstract: The functions of the somatosensory system are multiple. We use tactile input to localize and experience the various qualities of touch, and proprioceptive information to determine the position of different parts of the body with respect to each other, which provides fundamental information for action. Further, tactile exploration of the characteristics of external objects can result in conscious perceptual experience and stimulus or object recognition. Neuroanatomical studies suggest parallel processing as well as serial processing within the cerebral somatosensory system that reflect these separate functions, with one processing stream terminating in the posterior parietal cortex (PPC), and the other terminating in the insula. We suggest that, analogously to the organisation of the visual system, somatosensory processing for the guidance of action can be dissociated from the processing that leads to perception and memory. In addition, we find a second division between tactile information processing about external targets in service of object recognition and tactile information processing related to the body itself. We suggest the posterior parietal cortex subserves both perception and action, whereas the insula principally subserves perceptual recognition and learning. Keywords: body image; body schema; crossmodal; insula; parietal; proprioception; tactile object recognition 1. -

Nerves of the Orbit Optic Nerve the Optic Nerve Enters the Orbit from the Middle Cranial Fossa by Passing Through the Optic Canal
human anatomy 2016 lecture fourteen Dr meethak ali ahmed neurosurgeon Nerves of the Orbit Optic Nerve The optic nerve enters the orbit from the middle cranial fossa by passing through the optic canal . It is accompanied by the ophthalmic artery, which lies on its lower lateral side. The nerve is surrounded by sheath of pia mater, arachnoid mater, and dura mater. It runs forward and laterally within the cone of the recti muscles and pierces the sclera at a point medial to the posterior pole of the eyeball. Here, the meninges fuse with the sclera so that the subarachnoid space with its contained cerebrospinal fluid extends forward from the middle cranial fossa, around the optic nerve, and through the optic canal, as far as the eyeball. A rise in pressure of the cerebrospinal fluid within the cranial cavity therefore is transmitted to theback of the eyeball. Lacrimal Nerve The lacrimal nerve arises from the ophthalmic division of the trigeminal nerve. It enters the orbit through the upper part of the superior orbital fissure and passes forward along the upper border of the lateral rectus muscle . It is joined by a branch of the zygomaticotemporal nerve, whi(parasympathetic secretomotor fibers). The lacrimal nerve ends by supplying the skin of the lateral part of the upper lid. Frontal Nerve The frontal nerve arises from the ophthalmic division of the trigeminal nerve. It enters the orbit through the upper part of the superior orbital fissure and passes forward on the upper surface of the levator palpebrae superioris beneath the roof of the orbit . -

Regional Anesthesia in Head and Neck Surgery
TITLE: Regional Anesthesia in Head and Neck Surger SOURCE: Grand Rounds Presentation, UTMB, Dept. of Otolaryngology DATE: May 24, 2006 RESIDENT PHYSICIAN: Jacques Peltier, MD FACULTY PHYSICIAN: Francis B. Quinn, MD SERIES EDITORS: Francis B. Quinn, Jr., MD and Matthew W. Ryan, MD "This material was prepared by resident physicians in partial fulfillment of educational requirements established for the Postgraduate Training Program of the UTMB Department of Otolaryngology/Head and Neck Surgery and was not intended for clinical use in its present form. It was prepared for the purpose of stimulating group discussion in a conference setting. No warranties, either express or implied, are made with respect to its accuracy, completeness, or timeliness. The material does not necessarily reflect the current or past opinions of members of the UTMB faculty and should not be used for purposes of diagnosis or treatment without consulting appropriate literature sources and informed professional opinion." Introduction Local anesthetic techniques were popularized early in the history of surgery with the advent of injectable nerve blocking agents. Until their discovery, patients were either held down or knocked unconscious to perform procedures. In the early days of general anesthesia, local anesthesia was preferred in all cases that it was applicable due to the significant risks associated with general anesthesia. Many procedures performed today under general anesthesia, such as tonsillectomy, rhinoplasty, and even bronchoscopy, were performed under local anesthesia to avoid the perils of general anesthetics. With the introduction of pulse oximetry, safer inhaled anesthetics, and combined intravenous and inhaled general anesthesia techniques, general anesthesia has become much safer, resulting in many surgeons being unfamiliar with regional nerve blocks to perform surgery. -
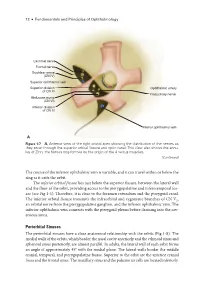
Periorbital Sinuses the Periorbital Sinuses Have a Close Anatomical Relationship with the Orbits (Fig 1-8)
12 ● Fundamentals and Principles of Ophthalmology Lacrimal nerve Frontal nerve Trochlear nerve (CN IV) Superior ophthalmic vein Superior division Ophthalmic artery of CN III Nasociliary nerve Abducens nerve (CN VI) Inferior division of CN III Inferior ophthalmic vein A Figure 1-7 A, Anterior view of the right orbital apex showing the distribution of the nerves as they enter through the superior orbital fissure and optic canal. This view also shows the annu- lus of Zinn, the fibrous ring formed by the origin of the 4 rectus muscles. (Continued) The course of the inferior ophthalmic vein is variable, and it can travel within or below the ring as it exits the orbit. The inferior orbital fissure lies just below the superior fissure, between the lateral wall and the floor of the orbit, providing access to the pterygopalatine and inferotemporal fos- sae (see Fig 1-1). Therefore, it is close to the foramen rotundum and the pterygoid canal. The inferior orbital fissure transmits the infraorbital and zygomatic branches of CN V2, an orbital nerve from the pterygopalatine ganglion, and the inferior ophthalmic vein. The inferior ophthalmic vein connects with the pterygoid plexus before draining into the cav- ernous sinus. Periorbital Sinuses The periorbital sinuses have a close anatomical relationship with the orbits (Fig 1-8). The medial walls of the orbits, which border the nasal cavity anteriorly and the ethmoid sinus and sphenoid sinus posteriorly, are almost parallel. In adults, the lateral wall of each orbit forms an angle of approximately 45° with the medial plane. The lateral walls border the middle cranial, temporal, and pterygopalatine fossae. -

Oscillatory Properties of Functional Connections Between Sensory Areas Mediate Cross-Modal Illusory Perception
The Journal of Neuroscience, July 17, 2019 • 39(29):5711–5718 • 5711 Systems/Circuits Oscillatory Properties of Functional Connections Between Sensory Areas Mediate Cross-Modal Illusory Perception Jason Cooke,1 Claudia Poch,2 Helge Gillmeister,1 Marcello Costantini,3,4 and XVincenzo Romei1,5 1Centre for Brain Science, Department of Psychology, University of Essex, Wivenhoe Park, Colchester CO4 3SQ, United Kingdom, 2Facultad de Lenguas y Educacio´n, Universidad Nebrija, 28015 Madrid, Spain, 3Department of Psychological, Health, and Territorial Sciences, 4Institute for Advanced Biomedical Technologies (ITAB), “G. d’Annunzio” University of Chieti-Pescara, 66100 Chieti, Italy, and 5Centro studi e ricerche in Neuroscienze Cognitive, Dipartimento di Psicologia, Universita’ di Bologna, Campus di Cesena, 47521 Cesena, Italy The presentation of simple auditory stimuli can significantly impact visual processing and even induce visual illusions, such as the auditory-induced double flash illusion (DFI). These cross-modal processes have been shown to be driven by occipital oscillatory activity within the alpha band. Whether this phenomenon is network specific or can be generalized to other sensory interactions remains unknown. The aim of the current study was to test whether cross-modal interactions between somatosensory-to-visual areas leading to the same (but tactile-induced) DFI share similar properties with the auditory DFI. We hypothesized that if the effects are mediated by the oscillatory properties of early visual areas per se, then the two versions of the illusion should be subtended by the same neurophysiolog- ical mechanism (i.e., the speed of the alpha frequency). Alternatively, if the oscillatory activity in visual areas predicting this phenomenon is dependent on the specific neural network involved, then it should reflect network-specific oscillatory properties. -

Anatomy-Nerve Tracking
INJECTABLES ANATOMY www.aestheticmed.co.uk Nerve tracking Dr Sotirios Foutsizoglou on the anatomy of the facial nerve he anatomy of the human face has received enormous attention during the last few years, as a plethora of anti- ageing procedures, both surgical and non-surgical, are being performed with increasing frequency. The success of each of those procedures is greatly dependent on Tthe sound knowledge of the underlying facial anatomy and the understanding of the age-related changes occurring in the facial skeleton, ligaments, muscles, facial fat compartments, and skin. The facial nerve is the most important motor nerve of the face as it is the sole motor supply to all the muscles of facial expression and other muscles derived from the mesenchyme in the embryonic second pharyngeal arch.1 The danger zone for facial nerve injury has been well described. Confidence when approaching the nerve and its branches comes from an understanding of its three dimensional course relative to the layered facial soft tissue and being aware of surface anatomy landmarks and measurements as will be discussed in this article. Aesthetic medicine is not static, it is ever evolving and new exciting knowledge emerges every day unmasking the relationship of the ageing process and the macroscopic and microscopic (intrinsic) age-related changes. Sound anatomical knowledge, taking into consideration the natural balance between the different facial structures and facial layers, is fundamental to understanding these changes which will subsequently help us develop more effective, natural, long-standing and most importantly, safer rejuvenating treatments and procedures. The soft tissue of the face is arranged in five layers: 1) Skin; 2) Subcutaneous fat layer; 3) Superficial musculoaponeurotic system (SMAS); 4) Areolar tissue or loose connective tissue (most clearly seen in the scalp and forehead); 5) Deep fascia formed by the periosteum of facial bones and the fascial covering of the muscles of mastication (lateral face). -

Anatomical Study of the Zygomaticotemporal Branch Inside the Orbit
Open Access Original Article DOI: 10.7759/cureus.1727 Anatomical Study of the Zygomaticotemporal Branch Inside the Orbit Joe Iwanaga 1 , Charlotte Wilson 1 , Koichi Watanabe 2 , Rod J. Oskouian 3 , R. Shane Tubbs 4 1. Seattle Science Foundation 2. Department of Anatomy, Kurume University School of Medicine 3. Neurosurgery, Complex Spine, Swedish Neuroscience Institute 4. Neurosurgery, Seattle Science Foundation Corresponding author: Charlotte Wilson, [email protected] Abstract The location of the opening of the zygomaticotemporal branch (ZTb) of the zygomatic nerve inside the orbit (ZTFIN) has significant surgical implications. This study was conducted to locate the ZTFIN and investigate the variations of the ZTb inside the orbit. A total of 20 sides from 10 fresh frozen cadaveric Caucasian heads were used in this study. The vertical distance between the inferior margin of the orbit and ZTFIN (V-ZTFIN), the horizontal distance between the lateral margin of the orbit and ZTFIN (H-ZTFIN), and the diameter of the ZTFIN (D-ZTFIN) were measured. The patterns of the ZTb inside the orbit were classified into five different groups: both ZTb and LN innervating the lacrimal gland independently (Group A), both ZTb and LN innervating the lacrimal gland with a communicating branch (Group B), ZTb joining the LN without a branch to the lacrimal gland (Group C), the ZTb going outside the orbit through ZTFIN without a branch to the lacrimal gland nor LN (Group D), and absence of the ZTb (Group E). The D-ZTFIN V-ZTFIN H-ZTFIN ranged from 0.2 to 1.1 mm, 6.6 to 21.5 mm, 2.0 to 11.3 mm, respectively. -

Oculoplastics/Orbit 2017-2019
Academy MOC Essentials® Practicing Ophthalmologists Curriculum 2017–2019 Oculoplastics and Orbit *** Oculoplastics/Orbit 2 © AAO 2017-2019 Practicing Ophthalmologists Curriculum Disclaimer and Limitation of Liability As a service to its members and American Board of Ophthalmology (ABO) diplomates, the American Academy of Ophthalmology has developed the Practicing Ophthalmologists Curriculum (POC) as a tool for members to prepare for the Maintenance of Certification (MOC) -related examinations. The Academy provides this material for educational purposes only. The POC should not be deemed inclusive of all proper methods of care or exclusive of other methods of care reasonably directed at obtaining the best results. The physician must make the ultimate judgment about the propriety of the care of a particular patient in light of all the circumstances presented by that patient. The Academy specifically disclaims any and all liability for injury or other damages of any kind, from negligence or otherwise, for any and all claims that may arise out of the use of any information contained herein. References to certain drugs, instruments, and other products in the POC are made for illustrative purposes only and are not intended to constitute an endorsement of such. Such material may include information on applications that are not considered community standard, that reflect indications not included in approved FDA labeling, or that are approved for use only in restricted research settings. The FDA has stated that it is the responsibility of the physician to determine the FDA status of each drug or device he or she wishes to use, and to use them with appropriate patient consent in compliance with applicable law. -

Cranial Neuralgias
CRANIAL NEURALGIAS Presented by: Neha Sharma M.D. Date: September 27th, 2019 TYPES OF NEURALGIAS ❖ TRIGEMINAL NEURALGIA ❖ GLOSSOPHARYNGEAL NEURALGIA ❖ NASOCILIARY NEURALGIA ❖ SUPERIOR LARYNGEAL NEURALGIA ❖ SUPRAORBITAL NEURALGIA ❖ OCCIPITAL NEURALGIA ❖ SPHENOPALATINE NEURALGIA ❖ GREAT AURICULAR NEURALGIA ❖ NERVUS INTERMEDIUS NEURALGIA ❖ TROCHLEAR NEURALGIA WHAT IS CRANIAL NEURALGIA? ❖ Paroxysmal pain of head, face and/or neck ❖ Unilateral sensory nerve distribution ❖ Pain is described as sharp, shooting, lancinating ❖ Primary or Secondary causes ❖ Multiple triggers TRIGEMINAL (CN V) NEURALGIA TRIGEMINAL NEURALGIA ❖ Also called Tic Douloureux ❖ Sudden, unilateral, electrical, shock-like, shooting, sharp pain. Presents affecting Cranial Nerve V; primarily V2 and V3 branches ❖ F>M; 3:1 TRIGEMINAL NEURALGIA ❖ Anatomy of Trigeminal Nerve ❖ Cranial Nerve V ❖ Three Branches: Ophthalmic, Maxillary and Mandibular ❖ Sensory supply to forehead/supraorbital, cheeks and jaw https://www.nf2is.org/cn5.php TRIGEMINAL NEURALGIA – TRIGGERS ❖ Mastication (73%) ❖ Eating (59%) ❖ Touch (69%) ❖ Talking (58%) ❖ Brushing Teeth (66%) ❖ Cold wind (50%) TYPES OF TRIGEMINAL NEURALGIA ❖ Primary/Classic/Idiopathic ❖ Vascular compression of the nerve – superior cerebellar artery ❖ Secondary/Symptomatic ❖ Caused by intracranial lesions ❖ Tumors, Strokes, Multiple Sclerosis (4%) ❖ Typical vs. Atypical ❖ Paroxysmal (79%) vs. Continuous (21%) IASP/IHS & CLASSIFICATIONS OF TRIGEMINAL NEURALGIA ❖ IASP – International Association ❖ Classifications for the Study of Pain ❖ I -

Clinical Anatomy of the Trigeminal Nerve
Clinical Anatomy of Trigeminal through the superior orbital fissure Nerve and courses within the lateral wall of the cavernous sinus on its way The trigeminal nerve is the fifth of to the trigeminal ganglion. the twelve cranial nerves. Often Ophthalmic Nerve is formed by the referred to as "the great sensory union of the frontal nerve, nerve of the head and neck", it is nasociliary nerve, and lacrimal named for its three major sensory nerve. Branches of the ophthalmic branches. The ophthalmic nerve nerve convey sensory information (V1), maxillary nerve (V2), and from the skin of the forehead, mandibular nerve (V3) are literally upper eyelids, and lateral aspects "three twins" carrying information of the nose. about light touch, temperature, • The maxillary nerve (V2) pain, and proprioception from the enters the middle cranial fossa face and scalp to the brainstem. through foramen rotundum and may or may not pass through the • The three branches converge on cavernous sinus en route to the the trigeminal ganglion (also called trigeminal ganglion. Branches of the semilunar ganglion or the maxillary nerve convey sensory gasserian ganglion), which contains information from the lower eyelids, the cell bodies of incoming sensory zygomae, and upper lip. It is nerve fibers. The trigeminal formed by the union of the ganglion is analogous to the dorsal zygomatic nerve and infraorbital root ganglia of the spinal cord, nerve. which contain the cell bodies of • The mandibular nerve (V3) incoming sensory fibers from the enters the middle cranial fossa rest of the body. through foramen ovale, coursing • From the trigeminal ganglion, a directly into the trigeminal single large sensory root enters the ganglion. -

Supraorbital Nerve Schwannoma in a Young Chinese Man: a Case Report
CASE REPORT Supraorbital nerve schwannoma in a young Chinese man: a case report and review of the literature Alison Yin-Yung Chan1, MBBS, Marcus M Marcet2, FCOphth HK, FHKAM (Ophth), Tiffany Wing-See Lau3, MBBS 1Hong Kong Eye Hospital 2Department of Ophthalmology, Hong Kong Sanatorium and Hospital, Hong Kong 3Department of Pathology, Queen Mary Hospital, Hong Kong Correspondence and reprint requests: Dr Alison Yin-Yung Chan, Hong Kong Eye Hospital. Email: [email protected] acuity. Confrontation testing revealed a left medial inferior Abstract quadrantanopia, which had been documented in 2008 as an old defect with optic atrophy, consistent with left optic disc Schwannomas of peripheral branches of the trigeminal pallor on fundi visualization. Ophthalmologic examination nerve are rare. We report a 23-year-old Chinese man was unremarkable. Systemic evaluation excluded neurofibromatosis. who underwent anterior orbitotomy through an upper eyelid crease incision approach for resection of a Contrast-enhanced computed tomography of the orbit supraorbital nerve schwannoma. Clinical features, demonstrated a well-circumscribed, homogenous, moderately imaging findings, and management considerations are enhancing ovoid solid mass of 1.2 cm × 0.8 cm × 0.8 cm discussed, and the literature reviewed. at the superomedial aspect of the extraconal left orbit, anterosuperior to and abutting the globe. There were no aggressive features (bony destruction or invasion to Key words: Eyelids; Neurilemmoma; Orbit surrounding structures), fluid, macroscopic fat component, or evidence of calcification. The left globe, lacrimal gland, Introduction and extraocular muscles were normal (Figure 1). Contrast- enhanced magnetic resonance imaging (MRI) showed a well- Schwannomas are benign, slow-growing tumors arising from defined, homogenous, T1-hypointense and T2-hyperintense, the myelin sheath Schwann cells of the peripheral nerves. -

Anatomy of the Periorbital Region Review Article Anatomia Da Região Periorbital
RevSurgicalV5N3Inglês_RevistaSurgical&CosmeticDermatol 21/01/14 17:54 Página 245 245 Anatomy of the periorbital region Review article Anatomia da região periorbital Authors: Eliandre Costa Palermo1 ABSTRACT A careful study of the anatomy of the orbit is very important for dermatologists, even for those who do not perform major surgical procedures. This is due to the high complexity of the structures involved in the dermatological procedures performed in this region. A 1 Dermatologist Physician, Lato sensu post- detailed knowledge of facial anatomy is what differentiates a qualified professional— graduate diploma in Dermatologic Surgery from the Faculdade de Medician whether in performing minimally invasive procedures (such as botulinum toxin and der- do ABC - Santo André (SP), Brazil mal fillings) or in conducting excisions of skin lesions—thereby avoiding complications and ensuring the best results, both aesthetically and correctively. The present review article focuses on the anatomy of the orbit and palpebral region and on the important structures related to the execution of dermatological procedures. Keywords: eyelids; anatomy; skin. RESU MO Um estudo cuidadoso da anatomia da órbita é muito importante para os dermatologistas, mesmo para os que não realizam grandes procedimentos cirúrgicos, devido à elevada complexidade de estruturas envolvidas nos procedimentos dermatológicos realizados nesta região. O conhecimento detalhado da anatomia facial é o que diferencia o profissional qualificado, seja na realização de procedimentos mini- mamente invasivos, como toxina botulínica e preenchimentos, seja nas exéreses de lesões dermatoló- Correspondence: Dr. Eliandre Costa Palermo gicas, evitando complicações e assegurando os melhores resultados, tanto estéticos quanto corretivos. Av. São Gualter, 615 Trataremos neste artigo da revisão da anatomia da região órbito-palpebral e das estruturas importan- Cep: 05455 000 Alto de Pinheiros—São tes correlacionadas à realização dos procedimentos dermatológicos.