Safety Evaluation Study Using Embryonic Stem (ES) Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sumitomo Chemical Estimate Total Approx
Create New Value Change and Innovation Create New Value MorganStanley MUFG Chemicals Conference December 19, 2017 1- 1 - Create New Value Contents Create New Value Performance Trends 3-9 Business Strategy 10-16 Business Strategy by Sector 17-51 Initiatives for Maintaining 52-61 Sustained Growth Conclusion 62-64 2- 2 - Performance Trends 3 Create New Value FY2017 1H vs. FY2016 1H (Billions of yen) FY2016 FY2017 1H 1H Change Sales 900.5 1,054.1 +153.6 Operating Income 47.3 92.0 +44.8 (Equity in Earnings of Affiliates) 18.8 22.6 +3.8 Ordinary Income 50.6 115.0 +64.4 Net Income Attributable to Owners of the Parents 19.2 68.5 +49.3 Naphtha Price ¥31,500/kl ¥37,600/kl Exchange Rate ¥105.20/$ ¥111.04/$ 4 Create New Value FY2017 1H vs. FY2016 1H: Operating Income by Sector (Billions of yen) FY2016 FY2017 Change Reason for Change 1H 1H Specialty Chemicals 45.4 72.5 +27.2 Energy & Functional Increased shipment volumes Materials 2.2 9.9 +7.7 of resorcinol and SEP Increased shipment volumes IT-related Chemicals 2.5 8.7 +6.2 of polarizing films and touchscreen panels Lower methionine market Health & Crop Sciences 12.9 5.6 -7.2 prices Pharmaceuticals 27.9 48.3 +20.5 Increased sales of Latuda Bulk Chemicals 6.7 25.0 +18.3 Petrochemicals & Improved margins of MMA Plastics 6.7 25.0 +18.3 and synthetic resins Others -4.8 -5.5 -0.8 Total 47.3 92.0 +44.8 5 Create New Value FY2017 Forecast vs. -

Sumitomo Chemical 100 Years Sumitomo Chemical 100 Years Published: August 2014 Published by Sumitomo Chemical Co.,Ltd
Sumitomo Chemical 100 Years Sumitomo Chemical 100 Years Published: August 2014 Published by Sumitomo Chemical Co.,Ltd. Production and editing : Sumitomo Chemical Co.,Ltd., Corporate History Editorial Office 27-1, Shinkawa 2-chome, Chuo-ku, Tokyo, Japan Production assisted by Dai Nippon Printing Co.,Ltd. Printed in Japan by Dai Nippon Printing Co.,Ltd. CONTENTS Chairman’s Message President’s Message The Path to a Global Chemical Company 1. The History of Sumitomo 8 2. History of Sumitomo Fertilizer Works 10 3. Going from a Fertilizer Manufacturer to a Chemical Company 14 4. Moving into Fine Chemicals 16 5. Post-war Recovery 18 6. Moving into the Agricultural Chemicals Business 20 7. Growth of the Pharmaceuticals Sector 22 8. Entering the Field of Petrochemicals 24 9. Dealing with the Oil Crises 26 10. Rise and Decline of Aluminum Business 28 11. Construction of Singapore Petrochemical Complex 32 12. Separation of Pharmaceutical Business from Sumitomo Chemical and Inauguration 34 of Dainippon Sumitomo Pharma Co., Ltd. 13. Expansion of Agricultural Chemicals Business 36 14. IT-related Chemicals Sector Established and Business Grows 38 15. Progress of the Rabigh Project 40 16. Aiming to be a Truly Global Chemical Company 42 17. For a Better Tomorrow 46 Reports from Regional Headquarters 48 Report from Beijing by Sumitomo Chemical (China) Co., Ltd. 48 Report from Singapore by Sumitomo Chemical (Asia Pacific) Pte Ltd 50 Report from Brussels by Sumitomo Chemical Europe S.A./N.V. 52 Report from New York by Sumitomo Chemical America, Inc. 54 The Sumitomo Spirit and Sumitomo Chemical’s Business Philosophy 56 Recent Sales and Profits 57 Sumitomo Chemical Locations in Japan 58 Major Companies of Sumitomo Chemical Group: Japan 59 Major Companies of Sumitomo Chemical Group: International 60 Chronology of Sumitomo Chemical 62 Chairman’s Message In October 2015, Sumitomo Chemical will cele- triggered by the fall of Lehman Brothers and the sub- brate its 100 year anniversary. -

SUMITOMO CHEMICAL COMPANY, LIMITED Notice of the 137 Ordinary General Meeting of Shareholders
SUMITOMO CHEMICAL COMPANY, LIMITED th Notice of the 137 Ordinary General Meeting of Shareholders THIS DOCUMENT IS A SUMMARY OF A NOTICE AND THE ATTACHMENTS THERETO, THE ORIGINALS OF WHICH ARE IN THE JAPANESE LANGUAGE. THIS DOCUMENT IS MADE ONLY FOR THE REFERENCE PURPOSES OF SHAREHOLDERS WHO LIVE IN COUNTRIES OUTSIDE OF JAPAN AND DOES NOT CONSTITUTE A FORMAL TRANSLATION OF THE ORIGINAL NOTICE AND THE ATTACHMENTS. IN THIS SUMMARY, CERTAIN INFORMATION IS INTENTIONALLY OMITTED. THE ORIGINALS CONTAIN MORE INFORMATION, SUCH AS THE REPORTS OF THE ACCOUNTING AUDITORS AND THE BOARD OF CORPORATE AUDITORS, ETC., WHILE SOME SUPPLEMENTAL INFORMATION NOT CONTAINED IN THE ORIGINALS IS ADDITIONALLY GIVEN IN THIS SUMMARY AS FOOTNOTES WITH ASTERISKS. To Our Shareholders: Please take notice that Sumitomo Chemical Company, Limited (the “Company”) has called an Ordinary General Meeting of Shareholders to be held in Tokyo, Japan on June 21, 2018 (the “Meeting”) for the following purposes: MATTERS TO BE REPORTED: No. 1. Reports on the Company’s business report, consolidated financial statements, and the results of both the Accounting Auditor’s and the Board of Corporate Auditors’ audits of the Company’s consolidated financial statements for the 137th fiscal period (from April 1, 2017, to March 31, 2018) (the “137th fiscal period”). No. 2. Reports on the Company’s non-consolidated financial statements for the 137th fiscal period. MATTERS TO BE RESOLVED: No. 1. To make a partial amendment to Articles of Incorporation. No. 2. To elect thirteen Directors. No. 3. To elect one Corporate Auditor. EXPLANATION OF THE SUBJECT MATTERS OF THE MEETING No. -

Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone?
IRLE IRLE WORKING PAPER #188-09 September 2009 Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone? James R. Lincoln, Masahiro Shimotani Cite as: James R. Lincoln, Masahiro Shimotani. (2009). “Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone?” IRLE Working Paper No. 188-09. http://irle.berkeley.edu/workingpapers/188-09.pdf irle.berkeley.edu/workingpapers Institute for Research on Labor and Employment Institute for Research on Labor and Employment Working Paper Series (University of California, Berkeley) Year Paper iirwps-- Whither the Keiretsu, Japan’s Business Networks? How Were They Structured? What Did They Do? Why Are They Gone? James R. Lincoln Masahiro Shimotani University of California, Berkeley Fukui Prefectural University This paper is posted at the eScholarship Repository, University of California. http://repositories.cdlib.org/iir/iirwps/iirwps-188-09 Copyright c 2009 by the authors. WHITHER THE KEIRETSU, JAPAN’S BUSINESS NETWORKS? How were they structured? What did they do? Why are they gone? James R. Lincoln Walter A. Haas School of Business University of California, Berkeley Berkeley, CA 94720 USA ([email protected]) Masahiro Shimotani Faculty of Economics Fukui Prefectural University Fukui City, Japan ([email protected]) 1 INTRODUCTION The title of this volume and the papers that fill it concern business “groups,” a term suggesting an identifiable collection of actors (here, firms) within a clear-cut boundary. The Japanese keiretsu have been described in similar terms, yet compared to business groups in other countries the postwar keiretsu warrant the “group” label least. -

Plastic Manufactures Manufacturer Tradename Material 3M Corp
Plastic Manufactures Manufacturer Tradename Material 3M Corp. Vikuiti PC/PET film 3M Performance Polymers FTPE Fluoroelastomer A. L. Hyde Company Hydlar PA aramid fiber reinforced A. Schulman Formion Ionomer A. Schulman Invision TPO A. Schulman Miraprene TPV A. Schulman Polyaxis Rotational molding compounds A. Schulman Polyfabs ABS A. Schulman Polyflam Flame retardant compounds A. Schulman Polyfort PP, PE, EVA A. Schulman Polyman ABS alloys A. Schulman Polypur PUR A. Schulman Polytrope TPO A. Schulman Polyvin Flexible PVC A. Schulman Schulablend ABS/PA A. Schulman Schuladur PBT A. Schulman Schulaflex Flexible elastomers A. Schulman Schulamid PA 6, PA 66 A. Schulman Schulink HDPE A. Schulman Sunprene PVC elastomer Achilles Corporation Achilles Foamed PS, PUR Aclo Compounders Accucomp Blended compounds Aclo Compounders Accuguard Flame retardant compounds Aclo Compounders Acculoy Alloyed resins Aclo Compounders Accutech Engineering resins Actiplast Acvitron Flexible PVC Addiplast Addilene PP Addiplast Addinyl PA 6, PA 66 Adell Adell Thermoplastic resin Advanced Elastomer Systems Dytron TPE Advanced Elastomer Systems Geolast TPV Advanced Elastomer Systems Santoprene EPTR Advanced Elastomer Systems Trefsin TPE Advanced Elastomer Systems VistaFlex TPO Advanced Elastomer Systems Vyram EPTR Advanced Polymer Alloys LLC DuraGrip Thermoplastic rubber Aiscondel Etinox PVC Akai Plastik Sanayi Ve Ticaret AS Aklamid PA 6, PA 66 Akai Plastik Sanayi Ve Ticaret AS Akmaril ABS Akai Plastik Sanayi Ve Ticaret AS Akron HIPS Albis Albis PA 6, PA 66 Albis Alcom PA 66, POM Albis Cellidor CAB, CP Albis Petlon PBT Albis Tedur PPS Alfagomma S.p.A. Alfater XL TPV Alfaline S.r.l. Alfacarb PC Alfaline S.r.l. Alfanyl PA 6, PA 66 Alfaline S.r.l. -

Facilitation of Information Transfer on Chemicals in Products
Facilitation of Information Transfer on Chemicals in Products The Ministry of Economy, Trade and Industry (METI) developed ‘chemSHERPA’ [kémʃéərpə] as a new information transfer scheme for chemicals in products throughout their supply chains. METI hopes that the dissemination of chemSHERPA may contribute to reduce the workload of both providers and recipients of the information. From the beginning of the development of chemSHERPA, METI has been in communication with international bodies such as the IEC and the IPC, etc., with the aim of developing chemSHERPA into not only a Japanese standard but also an International standard. To make it a de-facto standard, METI has introduced this scheme to international organizations and governments of other countries for their active use. The Joint Article Management Promotion Consortium (JAMP) is a governing body for chemSHERPA from April 2016 and see a shift to chemSHERPA. We believe many companies are preparing towards implementing chemSHERPA. Based on the efforts mentioned above, the following companies and company groups have agreed with the dissemination of chemSHERPA, and METI will continue to work with JAMP and companies to spread the use of chemSHERPA to internal as well as external supply chains as needed.(Please contact us if any company or company group has interest in putting its name below.) It should be noted, the use of the provision of data entry support tools is free of charge in principle with the aim of promoting wider use of chemSHERPA. [Contact information] Chemical Management Policy Division Manufacturing Industries Bureau Ministry of Economy, Trade and Industry [email protected] 03-3501-0080 (direct) 03-3501-1511 (ex. -

US5159004.Pdf
||||||||||||||| USOO559004A United States Patent (19) 11 Patent Number: 5,159,004 Furuta et al. (45. Date of Patent: Oct. 27, 1992 54, THERMOPLASTIC RESIN COMPOSITION 59-126460 7/1984 Japan. 75) Inventors: Motonobu Furuta, Tsukuba; Takashi OTHER PUBLICATIONS Maruyama, Kobe; Hiroyuki Harada, Kirk-Othmer Encyclopedia of Chemical Technology, Ichihara, all of Japan Third Edition, vol. 17. 73) Assignee: Sumitomo Chemical Company, Primary Examiner-Jacob Ziegler Limited, Osaka, Japan Attorney, Agent, or Firm-Birch, Stewart, Kolasch and 21 Appl. No.: 437,894 Birch 22 Filed: Nov. 17, 1989 57) ABSTRACT (30) Foreign Application Priority Data Disclosed herein is a thermoplastic resin composition Nov. 18, 1988 JP Japan ................................ 63-292080 which comprises: Dec. 15, 1988 JP Japan ............. ... 63-314863 (a) polyphenylene ether or a composition containing Mar. 13, 1989 JP Japan .................................. -060319 polyphenylene ether, 51 Int. Cl......................... C08K 5/01; C08K 51/04; (b) (i) a modified propylene polymer grafted with a C08K 53/02; C08K 71/12 styrene-based monomer or a mixture of a styrene 52 U.S. Cl. .................................... 524/390; 524/504; based monomer and a monomer copolymerizable 524/506; 524/508: 525/63; 525/68; 525/70, with the styrene-based monomer, or (ii) a composi 525/92; 525/93; 525/905 tion containing said modified propylene polymer and 58 Field of Search ..................... 525/68, 92,93, 905, a propylene polymer, 525/70; 524/508, 490, 504,506 (c) a rubbery substance, and at least one component selected from the following three components; 56 References Cited (d) a styrene resin having a melt index of 8 or above (at U.S. -
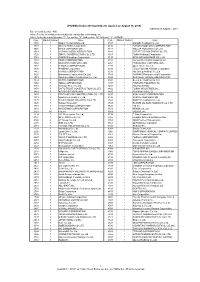
"JPX-Nikkei Index 400"
JPX-Nikkei Index 400 Constituents (applied on August 30, 2019) Published on August 7, 2019 No. of constituents : 400 (Note) The No. of constituents is subject to change due to de-listing. etc. (Note) As for the market division, "1"=1st section, "2"=2nd section, "M"=Mothers, "J"=JASDAQ. Code Market Divison Issue Code Market Divison Issue 1332 1 Nippon Suisan Kaisha,Ltd. 3107 1 Daiwabo Holdings Co.,Ltd. 1333 1 Maruha Nichiro Corporation 3116 1 TOYOTA BOSHOKU CORPORATION 1605 1 INPEX CORPORATION 3141 1 WELCIA HOLDINGS CO.,LTD. 1719 1 HAZAMA ANDO CORPORATION 3148 1 CREATE SD HOLDINGS CO.,LTD. 1720 1 TOKYU CONSTRUCTION CO., LTD. 3167 1 TOKAI Holdings Corporation 1721 1 COMSYS Holdings Corporation 3197 1 SKYLARK HOLDINGS CO.,LTD. 1801 1 TAISEI CORPORATION 3231 1 Nomura Real Estate Holdings,Inc. 1802 1 OBAYASHI CORPORATION 3254 1 PRESSANCE CORPORATION 1803 1 SHIMIZU CORPORATION 3288 1 Open House Co.,Ltd. 1808 1 HASEKO Corporation 3289 1 Tokyu Fudosan Holdings Corporation 1812 1 KAJIMA CORPORATION 3291 1 Iida Group Holdings Co.,Ltd. 1820 1 Nishimatsu Construction Co.,Ltd. 3349 1 COSMOS Pharmaceutical Corporation 1821 1 Sumitomo Mitsui Construction Co., Ltd. 3360 1 SHIP HEALTHCARE HOLDINGS,INC. 1824 1 MAEDA CORPORATION 3382 1 Seven & I Holdings Co.,Ltd. 1860 1 TODA CORPORATION 3391 1 TSURUHA HOLDINGS INC. 1861 1 Kumagai Gumi Co.,Ltd. 3401 1 TEIJIN LIMITED 1878 1 DAITO TRUST CONSTRUCTION CO.,LTD. 3402 1 TORAY INDUSTRIES,INC. 1881 1 NIPPO CORPORATION 3405 1 KURARAY CO.,LTD. 1893 1 PENTA-OCEAN CONSTRUCTION CO.,LTD. 3407 1 ASAHI KASEI CORPORATION 1911 1 Sumitomo Forestry Co.,Ltd. -

Polypropylene Compounds for Automotive Applications
Sumitomo Chemical Co., Ltd. Polypropylene Compounds for Petrochemicals Research Laboratory Automotive Applications Satoru MORITOMI Tsuyoshi WATANABE Susumu KANZAKI Engineering plastics and metals have been extensively replaced by polypropylene (PP) based materials in auto- motive parts in order to achieve weight reductions and cost savings. To accomplish this, PP compounds which are made from PP and other components are under intense investigation. In this paper, we review the progress of re- search into compounding technology, improvement of mechanical properties and functionalization. Some problems and solutions for injection molding are also summarized. Furthermore, material developments for environmental load reduction are shown. This paper is translated from R&D Report, “SUMITOMO KAGAKU”, vol. 2010-I. Introduction tion. Therefore, it is expected that PP consumption will continue to increase in the future. Because polypropylene (PP) is low in cost but has out- Fig. 1 shows an example of a general constitution of standing mechanical properties and moldability, it ac- PP compounds for automotive applications. The impact counts for more than half of all the plastic materials used strength of the “impact PP,” which is composed of homo in automobiles. PP compounds are used for a variety of PP and ethylene propylene copolymer (EP copolymer), parts, including bumper facias, instrumental panels and is improved by adding an ethylene-based elastomer such door trims. Several grades of PP compounds, with their as ethylene-butene or ethylene-octene copolymer, to diverse performance characteristics, have been devel- which inorganic filler such as talcum is added for en- oped by compounding PP with various other materials hanced rigidity. according to the performance requirements of the in- The growth of PP compounds for automotive applica- tended parts. -

Sumitomo Chemical Annual Report 2020 1 to Our Stakeholders
Annual Report 2020 Change and Innovation 3.0 For a Sustainable Future Contents 2 To Our Stakeholders 62 Value Creation Platform 64 Research and Development Introduction to Sumitomo Chemical 65 Intellectual Property 4 The History of Sumitomo Chemical 66 Digital Innovation 8 Flow of Value Creation 68 Addressing Climate Change 10 One Year at Sumitomo Chemical 70 Initiatives Towards 12 Financial and Non-Financial Highlights Building a Circular System for Plastics To Our Stakeholders 72 Respect for Human Rights Management Strategy 74 Human Resource Strategy 16 President’s Message Corporate Governance 22 Financial Strategy 76 Directors & Senior Management 24 Messages from Outside Directors 81 Corporate Governance 26 Our Sustainability Efforts 90 Compliance 30 Roundtable—Welcoming ESG Investors 91 Anti-corruption 34 FY2019-FY2021 Corporate Business Plan President’s Message 92 Responsible Care 38 Creating Value through Business 93 Dialogue with Shareholders and Investors 40 Each Sector Situation 94 External Evaluation 42 Petrochemicals & Plastics 46 Energy & Functional Materials 95 Corporate Data 50 IT-related Chemicals 96 Financial Review 54 Health & Crop Sciences 102 Consolidated Financial Statements 58 Pharmaceuticals 108 Corporate and Investor Information Roundtable —Welcoming ESG Investors Financial Statements in This Document Forward-looking Statements Beginning in fiscal 2017, the Sumitomo Chemical Group is adopt- Statements made in this annual report with respect to plans, strategies, and future performance ing international financial reporting standards (IFRS) in place of that are not historical facts are forward-looking statements involving risks and uncertainties. Japanese GAAP, which it previously used, and is therefore restating Sumitomo Chemical cautions that a number of factors could cause actual results to differ figures for the previous consolidated fiscal year using IFRS for materially from such statements including, but not limited to, general economic conditions in comparative analysis. -

METAL JAPAN You Can Enter All Concurrent Shows with This Ticket
This is a SAMPLE. Please request actual exhibition tickets from here. ▶▶▶ http://www.metal-japan.jp/en/inv/pre/ INVITATION TICKET Japan’s Largest*1 ! 170 Exhibitors Held inside Highly-functional Material Week 2017 50 Exhibitors Newly Exhibiting! 170 Exhibitors Gather 4th The numbers of exhibitors (including co-exhibitors), visitors and countries on this invitation ticket are forecast released on December 2, 2016. These numbers may differ from actual numbers at the show. *1 In highly-functional metal industry. *2 Including concurrent shows. *3 Including regions and concurrent shows. Covering All Advanced Metals & Technologies! Concurrent Shows 1,540 Exhibitors in total METAL JAPAN You can enter all concurrent shows with this ticket. East Hall 4–8 Floor Plan (Preliminary) METAL JAPAN Exhibitors ー Highly-functional Metal Expo ー 170 Highly-functional Material Week 850 Exhibitors Dates: April 5 [Wed] – 7 [Fri ] , 2017 10:00–18:00 (10:00–17:00 on Apr. 7) Cordially invited by: Organiser NEW Venue: Tokyo Big Sight, Japan Reed Exhibitions Japan Ltd. 2nd 4th 1st 6th 8th Office address: Organised by: Reed Exhibitions Japan Ltd. 18F Shinjuku-Nomura Bldg., Inspection/Analysis Processing Equipment Processing Technology CERAMICS METAL JOINING PLASTIC FilmTech 1-26-2 Nishishinjuku, Shinjuku-ku, Web: www.metal-japan.jp/en/ Tokyo 163-0570, Japan JAPAN JAPAN JAPAN JAPAN JAPAN • Non-destructive Inspection • Pressing Machine • Cutting Machine • Casting/Forging • Sheet Metal Working This ticket admits one person only. This exhibition is primarily open to trade. All visitors are required to bring an invitation ticket and • Material Analysis • Machining Center • Die-casting Machine • Die-casting • Powder Metallurgy 140 Exhibitors 170 Exhibitors 110 Exhibitors 180 Exhibitors 250 Exhibitors 2 business cards. -

Sumitomo Chemical Company Limited's Efforts Towards Chemical
Sumitomo Chemical Company Sumitomo Chemical Co., Ltd. Responsible Care Office Limited’s Efforts towards Tsuneo NARA* Chemical Management and Risk Communication The greatest feature of Sumitomo Chemical’s chemical management system is comprehensiveness based on our broad knowledge and technical ability as a chemical manufacturer. Furthermore, in order to practice risk communication, we are proactively holding dialogue with, and releasing information to, our diverse range of stakeholders, keeping in mind our motto “It is a mission for enterprises to grow together with the local commu- nity”. In February 2007 such efforts were finally acknowledged, when Sumitomo Chemical received the 2006 PRTR Grand Prize, the first time for a chemical company. Our company’s approach to chemical management and risk communication will be outlined in this document. This paper is translated from R&D Report, “SUMITOMO KAGAKU”, vol. 2007-II. Introduction ing lifestyle, and also to provide solutions to problems facing our society and the global environment. We Responsible care (RC) is an activity unique to each hope that continually promoting better CSR manage- enterprise for maintaining the environment, safety, ment activities will help us contribute to building more health and product quality over the whole life cycle of secure and sustainable development of society. its products and to deepen the trust and understanding received from other members of society, and at Sumito- Continuous Improvements in Proper Manage- mo Chemical Company Limited, we believe it is one of ment of Chemical Substances the most important pillars of our CSR management activities which extends to all of our factories and 1. Risk Management for Chemical Substances research centers in Japan and also to all Sumitomo We collect data and information on various types of group companies both within Japan and overseas.