Soil Surface Applications of Chemicals for the Control of Neonate Diaprepes Abbreviatus (Coleoptera: Curculionidae) and Their Effect on Ant Predators
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aalseth Aaron Aarup Aasen Aasheim Abair Abanatha Abandschon Abarca Abarr Abate Abba Abbas Abbate Abbe Abbett Abbey Abbott Abbs
BUSCAPRONTA www.buscapronta.com ARQUIVO 35 DE PESQUISAS GENEALÓGICAS 306 PÁGINAS – MÉDIA DE 98.500 SOBRENOMES/OCORRÊNCIA Para pesquisar, utilize a ferramenta EDITAR/LOCALIZAR do WORD. A cada vez que você clicar ENTER e aparecer o sobrenome pesquisado GRIFADO (FUNDO PRETO) corresponderá um endereço Internet correspondente que foi pesquisado por nossa equipe. Ao solicitar seus endereços de acesso Internet, informe o SOBRENOME PESQUISADO, o número do ARQUIVO BUSCAPRONTA DIV ou BUSCAPRONTA GEN correspondente e o número de vezes em que encontrou o SOBRENOME PESQUISADO. Número eventualmente existente à direita do sobrenome (e na mesma linha) indica número de pessoas com aquele sobrenome cujas informações genealógicas são apresentadas. O valor de cada endereço Internet solicitado está em nosso site www.buscapronta.com . Para dados especificamente de registros gerais pesquise nos arquivos BUSCAPRONTA DIV. ATENÇÃO: Quando pesquisar em nossos arquivos, ao digitar o sobrenome procurado, faça- o, sempre que julgar necessário, COM E SEM os acentos agudo, grave, circunflexo, crase, til e trema. Sobrenomes com (ç) cedilha, digite também somente com (c) ou com dois esses (ss). Sobrenomes com dois esses (ss), digite com somente um esse (s) e com (ç). (ZZ) digite, também (Z) e vice-versa. (LL) digite, também (L) e vice-versa. Van Wolfgang – pesquise Wolfgang (faça o mesmo com outros complementos: Van der, De la etc) Sobrenomes compostos ( Mendes Caldeira) pesquise separadamente: MENDES e depois CALDEIRA. Tendo dificuldade com caracter Ø HAMMERSHØY – pesquise HAMMERSH HØJBJERG – pesquise JBJERG BUSCAPRONTA não reproduz dados genealógicos das pessoas, sendo necessário acessar os documentos Internet correspondentes para obter tais dados e informações. DESEJAMOS PLENO SUCESSO EM SUA PESQUISA. -

2012 PSMFC Annual Report
Golden Gate Bridge, California 65th AnnuAl RepoRt of the pAcific StAteS MARine fiSheRieS coMMiSSion To the Congress of the United States, and to the Governors and Legislatures of the Five Compacting States — Washington, Oregon, California, Alaska and Idaho — 2012 Presented by the Commissioners of the Pacific States Marine Fisheries Commission in compliance with the State enabling acts creating the Commission and Public Laws 232; 766; and 315 of the 80th; 87th; and 91st Congresses of the United States assenting thereto. Respectfully submitted, PACIFIC STATES MARINE FISHERIES COMMISSION Randy Fisher, Executive Director Headquarters 205 SE Spokane Street, Suite 100 Portland, Oregon 97202-6413 2 | Pacific States Marine Fisheries Commission, 2012 Annual Report coMMiSSioneRS, ADViSoRS AnD cooRDinAtoRS 2012 State Commissioners Advisors Coordinator Alaska Bryce Edgmon Terry L. Johnson Karla Bush (ADFG) Eric A. Olson Don Lane Sue Aspelund Matthew Moir Gabe Sam Herman Savikko c California Charlton H. Bonham Jim Caito Marija Vojkovich (CDFG) o Thomas Harman Robert Fletcher MM Barbara Emley Donald K. Hansen i Mike McCorkle SS Aaron Newman ione Roger Thomas Kate Wing RS , A Idaho Virgil Moore Sharon Kiefer Pete Hassemer (IDFG) DV Eric Anderson Ed Schriever i S Fred Trevey Joe Stegner o RS Oregon Ed Bowles Wayne Butler Gway Kirchner (ODFW) A n Betsy Johnson Steve Fick D Jeff Feldner Liz Hamilton c Paul Heikkila oo Rod Moore RD Brad Pettinger Frank Warrens in A to Washington Phil Anderson Robert Alverson Heather Reed (WDFW) RS Brian Blake Mark Cedergreen Harriet A. Spanel Robert Jones Marion Larkin Irene Martin Bill Robinson Pacific States Marine Fisheries Commission, 2012 Annual Report | 3 MeSSAGe fRoM the eXecutiVe DiRectoR Randy Fisher, Executive Director It is a pleasure to provide the 2012 Annual Report of the Pacific States Marine Fisheries Commission. -
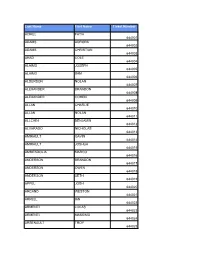
Last Name First Name Ticket Number ACIKEL FATIH 644001 ADAMS
Last Name First Name Ticket Number ACIKEL FATIH 644001 ADAMS AURORA 644002 ADAMS CHRISTIAN 644003 AHAD COLE 644004 ALAIMO JOSEPH 644005 ALAIMO SAM 644006 ALDERSON NOLAN 644007 ALEXANDER BRANDON 644008 ALEXANDER COHEN 644009 ALLAN CHARLIE 644010 ALLAN NOLAN 644011 ALLCHIN BENJAMIN 644012 ALVARADO NICHOLAS 644013 AMIRAULT GAVIN 644014 AMIRAULT JOSHUA 644015 AMMENDOLIA MARCO 644016 ANDERSON BRANDON 644017 ANDERSON OWEN 644018 ANDERSON SETH 644019 APPEL JOSH 644020 ARCAND WESTON 644021 ARKELL IAN 644022 ARMENTI LUCAS 644023 ARMENTI MASSIMO 644024 ARSENAULT TROY 644025 ASIS GRAYSON 644026 ASSELIN WYATT 644027 ATHERTON DATON 644028 AZEM SIMMONS BLAZE 644029 AZEM SIMMONS JADE 644030 AZEM SIMMONS KYLEN 644031 BACH AYDEN 644032 BAIOCCO MARIO 644033 BAIRD EVAN 644034 BAIRD LIAM 644035 BAIRD OWEN 644036 BAKER BENNETT 644037 BALL DANIEL 644038 BALL MATTHEW 644039 BANDERS LEO 644040 BANNISTER DYLAN 644041 BANNISTER LANDEN 644042 BARCHIESI BRYNN 644043 BARCHIESI SLOAN 644044 BARCLAY LONDON 644045 BARKER CHARLIE 644046 BARLOW HOLDEN 644047 BARNHARDT JAMES 644048 BARNHARDT WILLIAM 644049 BARRINGTON DYLAN 644050 BARRON LUCAS 644051 BARRY KELDON 644052 BARRY NOLAN 644053 BARRY TYSEN 644054 BARTON BROGAN 644055 BARTON NOLAN 644056 BASS-MELDRUM CALEB 644057 BAUGHMAN BRICE 644058 BAUGHMAN BRODY 644059 BEAMISH LEVI 644060 BEAMISH ZACK 644061 BEAUVAIS NOAH 644062 BEAVER BENJAMIN 644063 BEAVER JOSHUA 644064 BECK ERIC 644065 BELANGER BEAU 644066 BELANGER ETHAN 644067 BELFORD LINCOLN 644068 BENNETT COOPER 644069 BENSON JACK 644070 BERECZ MAX 644071 BERGEN CONNELL -

Intensively Monitored Watersheds: 2008 Fish Population Studies in the Hood Canal and Lower Columbia Stream Complexes
STATE OF WASHINGTON December 2009 Intensively Monitored Watersheds: 2008 Fish Population Studies in the Hood Canal and Lower Columbia Stream Complexes by Clayton Kinsel, Pat Hanratty, Mara Zimmerman, and Bryce Glaser, Steven Gray, Todd Hillson, Dan Rawding, Steven VanderPloeg Washington Department of FISH AND WILDLIFE Fish Program Science Division FPA 09-12 Intensively Monitored Watersheds: 2008 Fish Population Studies in the Hood Canal and Lower Columbia Stream Complexes Clayton Kinsel, Pat Hanratty, Mara Zimmerman and Bryce Glaser, Steven Gray, Todd Hillson, Dan Rawding, Steven VanderPloeg Fish Program Washington Department of Fish and Wildlife December 2009 Acknowledgements This work was funded by the Salmon Recovery Funding Board. Hood Canal Data for the Hood Canal IMW project were collected by an experienced crew of technicians led by Mat Gillum. Eric Kummerow, Scott Walker, and Karen Shields each brought their individual expertise, commitment, and enthusiasm to this project. They responded to the whim of the weather, tides, and fishing schedules and have intimate knowledge of the watersheds, the fish, and fishing seasons. All field staff put in long hours, often at night during inclement weather, to ensure that traps continued to fish and that fish were handled and sampled in a gentle and timely fashion. Pete Topping and Mike Ackley provided logistical support related to trap installation and removal and expertise in trap design and function. Kelly Kiyohara edited earlier versions of this report. Biologists from Weyerhauser and Washington Department of Ecology worked collaboratively to sample and mark coho parr. University of Washington has continuously provided access to the Big Beef property and research station since our long-term monitoring project began in 1978. -

Suncoast Grapevine
www.ficuswww.suncoastnps.org.usf.edu/orgs/suncoast The Suncoast Grapevine Newsletter of the Suncoast Native Plant Society, Inc. Volume 36 Number 2 February 2019 FEBRUARY CHAPTER MEETING --- At the Seminole Heights Library --- 4711 Central Ave. Tampa, Florida 33603-3905 Dispersion and Impacts of Texas Phoenix Palm decline on Sabal palmetto at the Golden Aster Preserve Presented by Chris Hanni Wednesday, February 20 at 7 PM Texas Phoenix Palm Decline (TPPD) is a new disease in Florida, first appearing between Tampa and Sarasota in 2006-2008. Hillsborough County has been an epicenter for the disease which has spread to 22 Florida counties and which affects several species of date palm as well as our state tree, the cabbage palm (Sabal palmetto). The disease is fatal and there is presently no cure. About the Speaker: Chris is a graduate student at the school of GeoSciences, University of South Florida. His goal is to increase awareness of the disease and how it’s impacting the Sabal palmetto (our state tree). Chris and his wife Rebekah are combat veterans (6 deployments). They have 2 children and have lived in Hills- borough County since 2007. He has an A.S. in Computer Science, a B.S. in Environmental Microbiology and a Masters in Geography (GIS and Spatial Analysis). He is planning a PhD in Geography and Environmental Science Policy to start this fall. In his spare time he writes music and works on his 1977 CJ5. Light refreshments will not be served at the library, a native plant donation auction follows the presentation. - submitted by Virginia Overstreet Note - The Suncoast Chapter’s Board of Directors has designated February’s regular monthly meeting to elect officers and the 2019 Board of Directors. -

Price's Scrub State Park
Price’s Scrub State Park Advisory Group Draft Unit Management Plan STATE OF FLORIDA DEPARTMENT OF ENVIRONMENTAL PROTECTION Division of Recreation and Parks September 2018 TABLE OF CONTENTS INTRODUCTION ...................................................................................1 PURPOSE AND SIGNIFICANCE OF THE PARK ....................................... 1 Park Significance ................................................................................1 PURPOSE AND SCOPE OF THE PLAN..................................................... 2 MANAGEMENT PROGRAM OVERVIEW ................................................... 7 Management Authority and Responsibility .............................................. 7 Park Management Goals ...................................................................... 8 Management Coordination ................................................................... 9 Public Participation ..............................................................................9 Other Designations .............................................................................9 RESOURCE MANAGEMENT COMPONENT INTRODUCTION ................................................................................. 11 RESOURCE DESCRIPTION AND ASSESSMENT..................................... 12 Natural Resources ............................................................................. 12 Topography .................................................................................. 12 Geology ...................................................................................... -

Lyonia Preserve Plant Checklist
Lyonia Preserve Plant Checklist Volusia County, Florida Aceraceae (Maple) Asteraceae (Aster) Red Maple Acer rubrum Bitterweed Helenium amarum Blackroot Pterocaulon virgatum Agavaceae (Yucca) Blazing Star Liatris sp. Adam's Needle Yucca filamentosa Blazing Star Liatris tenuifolia Nolina Nolina brittoniana Camphorweed Heterotheca subaxillaris Spanish Bayonet Yucca aloifolia Cudweed Gnaphalium falcatum Dog Fennel Eupatorium capillifolium Amaranthaceae (Amaranth) Dwarf Horseweed Conyza candensis Cottonweed Froelichia floridana False Dandelion Pyrrhopappus carolinianus Fireweed Erechtites hieracifolia Anacardiaceae (Cashew) Garberia Garberia heterophylla Winged Sumac Rhus copallina Goldenaster Pityopsis graminifolia Goldenrod Solidago chapmanii Annonaceae (Custard Apple) Goldenrod Solidago fistulosa Flag Paw paw Asimina obovata Goldenrod Solidago spp. Mohr's Throughwort Eupatorium mohrii Apiaceae (Celery) Ragweed Ambrosia artemisiifolia Dollarweed Hydrocotyle sp. Saltbush Baccharis halimifolia Spanish Needles Bidens alba Apocynaceae (Dogbane) Wild Lettuce Lactuca graminifolia Periwinkle Catharathus roseus Brassicaceae (Mustard) Aquifoliaceae (Holly) Poorman's Pepper Lepidium virginicum Gallberry Ilex glabra Sand Holly Ilex ambigua Bromeliaceae (Airplant) Scrub Holly Ilex opaca var. arenicola Ball Moss Tillandsia recurvata Spanish Moss Tillandsia usneoides Arecaceae (Palm) Saw Palmetto Serenoa repens Cactaceae (Cactus) Scrub Palmetto Sabal etonia Prickly Pear Opuntia humifusa Asclepiadaceae (Milkweed) Caesalpinceae Butterfly Weed Asclepias -

Biology and Distribution of Butterfly Fauna of Hazara University, Garden Campus, Mansehra, Pakistan
Vol.3, No.2A, 28-36 (2013) Open Journal of Animal Sciences http://dx.doi.org/10.4236/ojas.2013.32A004 Biology and distribution of butterfly fauna of Hazara University, Garden Campus, Mansehra, Pakistan Farzana Perveen*, Fatima Fazal Department of Zoology, Shaheed Benazir Bhutto University (SBBU), Main Campus, Sheringal, Pakistan; *Corresponding Author: [email protected] Received 18 April 2013; revised 21 May 2013; accepted 30 May 2013 Copyright © 2013 Farzana Perveen, Fatima Fazal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT 1. INTRODUCTION The butterflies are beautiful creature of nature Lepidoptera have significant economic importance. with great economic importance as pollinator as Butterflies are the most efficient pollinators of flowers in well as bio-indicator of environments. The pre- addition to moths and bees. They help in production of sent survey was conducted to determine the food crops, seeds and fruits, therefore, they are essential biology and distribution of butterfly fauna of for the survival of man and animals [1]. Mouth parts of a Hazara University, Garden Campus, Mansehra, butterfly are adapted for sucking. Proboscis is usually Pakistan during March-June 2012. The study long and coiled. Compound eyes are comparatively large area was divided into 3 quadrates, i.e., residen- with a large number of facets. Larva is called caterpillar, tial area, administration area and main campus. usually eruciformes with a well-developed head. Cater- A total of 170 specimens were collected, 10 spe- pillar has well developed silk glands [2]. -

Nomenclatural Studies Toward a World List of Diptera Genus-Group Names
Nomenclatural studies toward a world list of Diptera genus-group names. Part V Pierre-Justin-Marie Macquart Evenhuis, Neal L.; Pape, Thomas; Pont, Adrian C. DOI: 10.11646/zootaxa.4172.1.1 Publication date: 2016 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Evenhuis, N. L., Pape, T., & Pont, A. C. (2016). Nomenclatural studies toward a world list of Diptera genus- group names. Part V: Pierre-Justin-Marie Macquart. Magnolia Press. Zootaxa Vol. 4172 No. 1 https://doi.org/10.11646/zootaxa.4172.1.1 Download date: 02. Oct. 2021 Zootaxa 4172 (1): 001–211 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Monograph ZOOTAXA Copyright © 2016 Magnolia Press ISSN 1175-5334 (online edition) http://doi.org/10.11646/zootaxa.4172.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:22128906-32FA-4A80-85D6-10F114E81A7B ZOOTAXA 4172 Nomenclatural Studies Toward a World List of Diptera Genus-Group Names. Part V: Pierre-Justin-Marie Macquart NEAL L. EVENHUIS1, THOMAS PAPE2 & ADRIAN C. PONT3 1 J. Linsley Gressitt Center for Entomological Research, Bishop Museum, 1525 Bernice Street, Honolulu, Hawaii 96817-2704, USA. E-mail: [email protected] 2 Natural History Museum of Denmark, Universitetsparken 15, 2100 Copenhagen, Denmark. E-mail: [email protected] 3Oxford University Museum of Natural History, Parks Road, Oxford OX1 3PW, UK. E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by D. Whitmore: 15 Aug. 2016; published: 30 Sept. 2016 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 NEAL L. -

6. Bremsen Als Parasiten Und Vektoren
DIPLOMARBEIT / DIPLOMA THESIS Titel der Diplomarbeit / Title of the Diploma Thesis „Blutsaugende Bremsen in Österreich und ihre medizini- sche Relevanz“ verfasst von / submitted by Manuel Vogler angestrebter akademischer Grad / in partial fulfilment of the requirements for the degree of Magister der Naturwissenschaften (Mag.rer.nat.) Wien, 2019 / Vienna, 2019 Studienkennzahl lt. Studienblatt / A 190 445 423 degree programme code as it appears on the student record sheet: Studienrichtung lt. Studienblatt / Lehramtsstudium UF Biologie und Umweltkunde degree programme as it appears on UF Chemie the student record sheet: Betreut von / Supervisor: ao. Univ.-Prof. Dr. Andreas Hassl Danksagung Hiermit möchte ich mich sehr herzlich bei Herrn ao. Univ.-Prof. Dr. Andreas Hassl für die Vergabe und Betreuung dieser Diplomarbeit bedanken. Seine Unterstützung und zahlreichen konstruktiven Anmerkungen waren mir eine ausgesprochen große Hilfe. Weiters bedanke ich mich bei meiner Mutter Karin Bock, die sich stets verständnisvoll ge- zeigt und mich mein ganzes Leben lang bei all meinen Vorhaben mit allen ihr zur Verfügung stehenden Kräften und Mitteln unterstützt hat. Ebenso bedanke ich mich bei meiner Freundin Larissa Sornig für ihre engelsgleiche Geduld, die während meiner zahlreichen Bremsenjagden nicht selten auf die Probe gestellt und selbst dann nicht überstrapaziert wurde, als sie sich während eines Ausflugs ins Wenger Moor als ausgezeichneter Bremsenmagnet erwies. Auch meiner restlichen Familie gilt mein Dank für ihre fortwährende Unterstützung. -

Aphids (Hemiptera, Aphididae)
A peer-reviewed open-access journal BioRisk 4(1): 435–474 (2010) Aphids (Hemiptera, Aphididae). Chapter 9.2 435 doi: 10.3897/biorisk.4.57 RESEARCH ARTICLE BioRisk www.pensoftonline.net/biorisk Aphids (Hemiptera, Aphididae) Chapter 9.2 Armelle Cœur d’acier1, Nicolas Pérez Hidalgo2, Olivera Petrović-Obradović3 1 INRA, UMR CBGP (INRA / IRD / Cirad / Montpellier SupAgro), Campus International de Baillarguet, CS 30016, F-34988 Montferrier-sur-Lez, France 2 Universidad de León, Facultad de Ciencias Biológicas y Ambientales, Universidad de León, 24071 – León, Spain 3 University of Belgrade, Faculty of Agriculture, Nemanjina 6, SER-11000, Belgrade, Serbia Corresponding authors: Armelle Cœur d’acier ([email protected]), Nicolas Pérez Hidalgo (nperh@unile- on.es), Olivera Petrović-Obradović ([email protected]) Academic editor: David Roy | Received 1 March 2010 | Accepted 24 May 2010 | Published 6 July 2010 Citation: Cœur d’acier A (2010) Aphids (Hemiptera, Aphididae). Chapter 9.2. In: Roques A et al. (Eds) Alien terrestrial arthropods of Europe. BioRisk 4(1): 435–474. doi: 10.3897/biorisk.4.57 Abstract Our study aimed at providing a comprehensive list of Aphididae alien to Europe. A total of 98 species originating from other continents have established so far in Europe, to which we add 4 cosmopolitan spe- cies of uncertain origin (cryptogenic). Th e 102 alien species of Aphididae established in Europe belong to 12 diff erent subfamilies, fi ve of them contributing by more than 5 species to the alien fauna. Most alien aphids originate from temperate regions of the world. Th ere was no signifi cant variation in the geographic origin of the alien aphids over time. -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000).