This Is Normal Text
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
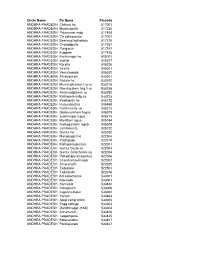
Post Offices
Circle Name Po Name Pincode ANDHRA PRADESH Chittoor ho 517001 ANDHRA PRADESH Madanapalle 517325 ANDHRA PRADESH Palamaner mdg 517408 ANDHRA PRADESH Ctr collectorate 517002 ANDHRA PRADESH Beerangi kothakota 517370 ANDHRA PRADESH Chowdepalle 517257 ANDHRA PRADESH Punganur 517247 ANDHRA PRADESH Kuppam 517425 ANDHRA PRADESH Karimnagar ho 505001 ANDHRA PRADESH Jagtial 505327 ANDHRA PRADESH Koratla 505326 ANDHRA PRADESH Sirsilla 505301 ANDHRA PRADESH Vemulawada 505302 ANDHRA PRADESH Amalapuram 533201 ANDHRA PRADESH Razole ho 533242 ANDHRA PRADESH Mummidivaram lsg so 533216 ANDHRA PRADESH Ravulapalem hsg ii so 533238 ANDHRA PRADESH Antarvedipalem so 533252 ANDHRA PRADESH Kothapeta mdg so 533223 ANDHRA PRADESH Peddapalli ho 505172 ANDHRA PRADESH Huzurabad ho 505468 ANDHRA PRADESH Fertilizercity so 505210 ANDHRA PRADESH Godavarikhani hsgso 505209 ANDHRA PRADESH Jyothinagar lsgso 505215 ANDHRA PRADESH Manthani lsgso 505184 ANDHRA PRADESH Ramagundam lsgso 505208 ANDHRA PRADESH Jammikunta 505122 ANDHRA PRADESH Guntur ho 522002 ANDHRA PRADESH Mangalagiri ho 522503 ANDHRA PRADESH Prathipadu 522019 ANDHRA PRADESH Kothapeta(guntur) 522001 ANDHRA PRADESH Guntur bazar so 522003 ANDHRA PRADESH Guntur collectorate so 522004 ANDHRA PRADESH Pattabhipuram(guntur) 522006 ANDHRA PRADESH Chandramoulinagar 522007 ANDHRA PRADESH Amaravathi 522020 ANDHRA PRADESH Tadepalle 522501 ANDHRA PRADESH Tadikonda 522236 ANDHRA PRADESH Kd-collectorate 533001 ANDHRA PRADESH Kakinada 533001 ANDHRA PRADESH Samalkot 533440 ANDHRA PRADESH Indrapalem 533006 ANDHRA PRADESH Jagannaickpur -

{Download PDF} the Formation of the Colonial State in India 1St Edition
THE FORMATION OF THE COLONIAL STATE IN INDIA 1ST EDITION PDF, EPUB, EBOOK Tony Cleaver | 9781134494293 | | | | | The Formation of the Colonial State in India 1st edition PDF Book Additionally, several Indian Princely States provided large donations to support the Allied campaign during the War. Under the charter, the Supreme Court, moreover, had the authority to exercise all types of jurisdiction in the region of Bengal, Bihar, and Odisha, with the only caveat that in situations where the disputed amount was in excess of Rs. During this age India's economy expanded, relative peace was maintained and arts were patronized. Routledge Handbook of Gender in South Asia. British Raj. Two four anna stamps issued in Description Contents Reviews Preview "Colonial and Postcolonial Geographies of India offers a good introduction to and basis for rethinking the ways in which academics theorize and teach the geographies of peoples, places, and regions. Circumscription theory Legal anthropology Left—right paradigm State formation Political economy in anthropology Network Analysis and Ethnographic Problems. With the constituting of the Ceded and Conquered Provinces in , the jurisdiction would extend as far west as Delhi. Contracts were awarded in to the East Indian Railway Company to construct a mile railway from Howrah -Calcutta to Raniganj ; to the Great Indian Peninsular Railway Company for a service from Bombay to Kalyan , thirty miles away; and to the Madras Railway Company for a line from Madras city to Arkonam , a distance of some thirty nine miles. The interdisciplinary work throws new light on pressing contemporary issues as well as on issues during the colonial period. -

Government Polytechnic Gajendragad
+91-9902609406 Government Polytechnic Gajendragad https://www.indiamart.com/government-polytechnic-gajendragad/ The Government Polytechnic, Gajendragad had been established by Government of Karnataka in the year 2007-08 vide GO No:ED:174:yoyok2007 dated: 23rd July 2007(ED:107:sweemer(Unique) 2007)to meet the needs of meritorious rural students. This ... About Us The Government Polytechnic, Gajendragad had been established by Government of Karnataka in the year 2007-08 vide GO No:ED:174:yoyok2007 dated: 23rd July 2007(ED:107:sweemer(Unique) 2007)to meet the needs of meritorious rural students. This institution is imparting quality technical education to meritorious rural youth. This polytechnic exploits the rural talents through technical education and need based training programmes. Our institution is located on Hubli -Hyderabad State Highway no. 06 in Gadag District. Gajendragad is a small town with a population of 40,000 in Ron Taluk of Gadag district. The main occupation of people is agriculture and weaving. Many granite industries exist in and around Gajendragad. This Institution was approved by AICTE New Delhi vide letter No: 770-53-220/NDIP/2007/SWRO dated 1st May 2008 with the following Engineering courses with sanctioned intake of 40 per course. 1. Diploma in Civil Engineering. 2.Diploma in Mechanical Engineering. 3.Diploma in Electronics and Communication Engineering. 4. Diploma in Computer Science and Engineering. The intake for the above Engineering courses was enhanced to 60 by AICTE, New Delhi vide letter No:770-53-220/NDIP/2007/SWRO dated 1st of June 2009. The MHRD,New Delhi has sanctioned administrative approval to start Community Development through Polytechnic scheme in 2009-10 The institution has well established building and workshop in a sprawling area.. -

Shivaji the Great
SHIVAJI THE GREAT BY BAL KRISHNA, M. A., PH. D., Fellow of the Royal Statistical Society. the Royal Economic Society. London, etc. Professor of Economics and Principal, Rajaram College, Kolhapur, India Part IV Shivaji, The Man and His .Work THE ARYA BOOK DEPOT, Kolhapur COPYRIGHT 1940 the Author Published by The Anther A Note on the Author Dr. Balkrisbna came of a Ksbatriya family of Multan, in the Punjab* Born in 1882, be spent bis boyhood in struggles against mediocrity. For after completing bis primary education he was first apprenticed to a jewel-threader and then to a tailor. It appeared as if he would settle down as a tailor when by a fortunate turn of events he found himself in a Middle Vernacular School. He gave the first sign of talents by standing first in the Vernacular Final ^Examination. Then he joined the Multan High School and passed en to the D. A. V. College, Lahore, from where he took his B. A* degree. Then be joined the Government College, Lahore, and passed bis M. A. with high distinction. During the last part of bis College career, be came under the influence of some great Indian political leaders, especially of Lala Lajpatrai, Sardar Ajitsingh and the Honourable Gopal Krishna Gokhale, and in 1908-9 took an active part in politics. But soon after he was drawn more powerfully to the Arya Samaj. His high place in the M. A. examination would have helped him to a promising career under the Government, but he chose differently. He joined Lala Munshiram ( later Swami Shraddha- Btnd ) *s a worker in the Guruk.ul, Kangri. -

New Vtp Applicants List
Contact Person Date of Name Address City District PinCode Telephone Mobile Email Contact Person Name VTP CP Email Mobile Application RURAL DEVELOPMENT AND TRAINING SRIRANGA nithyananda_mv@yah OPP SBM BANK, MAIN ROAD Mandya 571438 08236-252334 9845446401 [email protected] NITHYANANDA MV 9845446401 15-Apr-15 SOCIETY(R) PATNA oo.in RURAL DEVELOPMENT AND TRAINING SRIRANGA nithyananda_mv@yah OPP SBM BANK, MAIN ROAD Mandya 571438 08236-252334 9845446401 [email protected] NITHYANANDA MV 9845446401 15-Apr-15 SOCIETY(R) PATNA oo.in BENGALU [email protected] RACHANA ENTERPRISES PLOT NO-15, ABOVE CORPORATION BANK, KENGARI Bangalore 560074 080-28437482 9620400770 [email protected] UMA RUDRESH 9972920022 15-Apr-15 RU m # 2934/25 E 2ND FLOOR ABOVE HDFC BANK CLUB ROAD BANGALO [email protected] raghunathv@sriakshay SRI AKSHAY TECHNOLOGIES Bangalore 560040 080-41493098 9739011252 RAGHUNATHA.V 9739011252 15-Apr-15 VIJAYANAGAR RE m tech.com # 2934/25 E 2ND FLOOR ABOVE HDFC BANK CLUB ROAD BANGALO [email protected] raghunathv@sriakshay SRI AKSHAY TECHNOLOGIES Bangalore 560040 080-41493098 9739011252 RAGHUNATHA.V 9739011252 15-Apr-15 VIJAYANAGAR RE m tech.com RURAL DEVELOPMENT AND TRAINING SRIRANGA nithyananda_mv@yah OPP. SBM BANK , MAIN ROAD Mandya 571438 08236-252334 9845446401 [email protected] NITHYANANDA M V 9845446401 15-Apr-15 SOCIETY(R) PATNA oo.in # 2934/25 E 2ND FLOOR ABOVE HDFC BANK CLUB ROAD BANGALO [email protected] raghunathv@sriakshay SRI AKSHAY TECHNOLOGIES Bangalore 560040 080-41493098 9739011252 RAGHUNATHA.V -
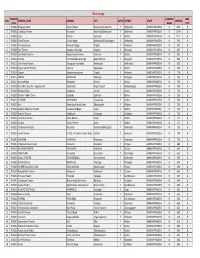
Real Image THEATRE COMPANY WEB S.No
Real Image THEATRE COMPANY WEB S.No. THEATRE_NAME ADDRESS CITY ACTIVE DISTRICT STATE SEATING CODE NAME CODE 1 TH0001 Bhujanga 70mm Shapure Nagar Hyderabad (Jedimetla) Y Hyderabad ANDHRA PRADESH RI 1311 0 2 TH0002 Laxmikala Theatre Moosapet Hyderabad(Moosapet) Y Hyderabad ANDHRA PRADESH RI 1189 0 3 TH0003 Sarat Krishna Gudivada Y Krishna ANDHRA PRADESH RI 1112 0 4 TH0006 Geeta Theatre Chanda Nagar Hyderabad (Chandanagar) Y Hyderabad ANDHRA PRADESH RI 962 0 5 TH0007 Srinivasa Deluxe Mahendra Nagar Ongole Y Prakasam ANDHRA PRADESH RI 900 0 6 TH0009 Devi Theatre Janagoan, Warangal Jangaon Y Warangal ANDHRA PRADESH RI 837 0 7 TH0010 Sree Balaji Theatres Satyanarayana Puram Gudivada Y Krishna ANDHRA PRADESH RI 833 0 8 TH0011 Ashoka Hanumakonda, Warangal Hanumakonda Y Warangal ANDHRA PRADESH RI 830 0 9 TH0012 Sree Prema Theatre Tukkuguda Hyderabad Hyderabad Y Hyderabad ANDHRA PRADESH RI 800 0 10 TH0013 Vijaya Lakshmi Theatre Kaanuru Vijayawada Y Krishna ANDHRA PRADESH RI 786 0 11 TH0014 Satyam Satyanarayanapuram Ongole Y Prakasam ANDHRA PRADESH RI 779 0 12 TH0015 NATRAJ WARANGAL Warangal Y Warangal ANDHRA PRADESH RI 766 0 13 TH0017 Krishna Mahal Kothapeta Guntur Y Guntur ANDHRA PRADESH RI 750 0 14 TH0018 Ravi 70mm A/c DTS - Nagarkarnool Sripur Road Nagarkarnool Y Mehboobnagar ANDHRA PRADESH RI 750 0 15 TH0019 Bhaskar Palace Kothapeta Guntur Y Guntur ANDHRA PRADESH RI 740 0 16 TH0020 Bhaskar Talkies 70mm Gudivada Gudivada Y Krishna ANDHRA PRADESH RI 738 0 17 TH0021 ALANKAR VIJAYAWADA Vijayawada Y Krishna ANDHRA PRADESH RI 738 0 18 TH0022 Ravi -

World Bank Document
Report No. 32253-IN INDIA Urban Finance and Governance Review Public Disclosure Authorized (In Two Volumes) Volume II: Case Study Annexes December 2004 Energy and Infrastructure Unit South Asia Region Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Document of the World Bank Vol. II - Case Study Annexes CURRENCY EQUIVALENTS Currency unit: Indian Rupee (RS) US$1 = Rs. 44.66 GOVERNMENT FISCAL YEAR April 1- March 31 ABBREVIATIONS AND ACRONYMS BATF Bangalore Agenda Task Force KAS Kamataka Administrative Service BDA Bangalore Metropolitan Region K-HB Karnataka Housing Board Development Authority KMA Karnataka Municipalities Act BMC Brihan Mumbai Corporation KMAS Kamataka Municipal Administrative ("Mumbai") Service BMP Bangalore City Corporation KMCA Kamataka Municipal Corporation BWSSB Bangalore Water Supply and Act Sewerage Board KTCP Karnataka Town and Country CAA Constitution Amendment Act Planning Act CAS Country Assistance Strategy KUIDFC Kamataka Urban Infrastructure CC City Corporations (Karnataka) Development Finance Corporation CCF City Challenge Fund KUWSDB Karnataka Urban Water Supply and CMC City Municipal Councils Drainage Board (Karnataka) LG Local Government CMWSSB Chennai Metropolitan Water Supply MDF Municipal Development Fund & Sewerage Board NGO Non-Governmental Organization DA Development Authorities PWD Public Works Department DFID Department for International SCB Slum Clearance Board Development SFC StateFinanceCommissions DMA Directorate of Municipal SWM Solid Waste Management Administration TA Technical Assistance EFC Eleventh Finance Commission TAC TechMnicalAssstnc l ESW Economic Sector Work (KanMataka) GDP Gross Domestic Product TNUDF Taril Nadu Urban Development GOI Government of India Fund GOK Government of Kamataka Fund GOM Government of Maharashtra TP Town Panchayats GOTN Government of Tamil Nadu ULDD Urban Development Department HDFC Housing Development Finance LB Urban Noa Body CorporationCopoatonLt., Ltd. -

Responsible for Plague in Bombay Province, Though They Have Been
Bull. Org. mond. Sante Bull. World Hlth Org.J 1951, 4, 75-109 SPREAD OF PLAGUE IN THE SOUTHERN AND CENTRAL DIVISIONS OF BOMBAY PROVINCE AND PLAGUE ENDEMIC CENTRES IN THE INDO-PAKISTAN SUBCONTINENT a M. SHARIF, D.Sc., Ph.D., F.N.I. Formerly Assistant Director in Charge of Department of Entomology, Haffkine Institute, Bombay b Manuscript received in September 1949 The findings of the Plague Recrudescence Inquiry in Sholapur and Adjoining Districts, conducted by Sharif & Narasimham11 12 in the districts of Sholapur and Dharwar during 1940 to 1943, do not support the idea that wild rodents help to carry plague infection from one place to another as in " temperate climes ".4 Wild rodents cannot be considered responsible for plague in Bombay Province, though they have been shown to be so in Transbaikalia, Mongolia, South-Eastern Russia, South Africa, and the western parts of the USA.17 In Bombay Province, the domestic rat perpetuates the plague infection. In some suitable places the infection among domestic rats goes on throughout the year. The infection is not apparent during the hot and dry season, its intensity being diminished because of the ill effect of prevailing climatic conditions on the wanderings of adult rat-fleas ; it pursues the course of a slow subterranean enzootic from burrow to burrow. The conclusion of the off-season is characterized by the advent of the rainy season, which exerts its influence in two ways first, it causes the rats from outside shelters to herd into burrows indoors and remain there perforce, which results in a considerable increase in the rat population within houses; secondly, it brings down the temperature and increases the humidity to such an extent as to result in a striking rise in the flea population and to allow rat-fleas to come out of burrows to attack human beings. -

Sl No District CVC Name Category 1 Gadag Aashraya Hospital Private 2
ಕ ೋ풿蓍 ಲಕಾಕರಣ ಕ ೋᲂ飍ರಗಳು (COVID VACCINATION CENTRES) Sl No District CVC Name Category 1 Gadag Aashraya Hospital Private 2 Gadag Abbigeri P3 Government 3 Gadag Adavisomapur - SUBCENTER Government 4 Gadag ANTUR - SUBCENTER Government 5 Gadag Bagewadi PHC P3 Government 6 Gadag Balaganur - SUBCENTER Government 7 Gadag Balehosur COVAXIN Government 8 Gadag Balehosur PHC2.0 Government 9 Gadag Balehosur Sub Centre Government 10 Gadag Banahatti SUBCENTERN Government 11 Gadag BELADADI- A- SUBCENTER Government 12 Gadag Belavanki P3 Government 13 Gadag Bellatti COVAXIN Government 14 Gadag Bellatti PHC2.0 Government 15 Gadag Bellatti Sub Centre Government 16 Gadag BETAGERI PHC COWAXIN Government 17 Gadag Betageri PHC-2.0 Government 18 Gadag BETAGERI UPHC COWAXIN Government 19 Gadag Betageri UPHC-2.0 Government 20 Gadag Chikkahandigol - SUBCENTER Government 21 Gadag CHIKKANARAGUND PHc Government 22 Gadag Chikkanaragund SUBCENTERN Government 23 Gadag Chikknargund Covaxin Government 24 Gadag CHINCHALI COWAXIN Government 25 Gadag Chinchali PHC -2.0 Government 26 Gadag CSI Hospital Betageri Private 27 Gadag Dambal PHC P3 Government 28 Gadag Dr. N B Patil Hospital Private 29 Gadag DUNDUR - SUBCENTER Government 30 Gadag GADAG GIMS Government 31 Gadag Gadag Uphc-2.0 Government 32 Gadag Gajendragad P3 Government 33 Gadag GDG-COVAXIN 1 Government 34 Gadag GIMS COVAXIN Government 35 Gadag GIMS-2.0- 2nd CVC Government 36 Gadag GIMS-2.0- 3rd CVC Government 37 Gadag GIMS-2.0- 4rth CVC Government 38 Gadag GIMS-2.0- 5th CVC Government 39 Gadag Hadli A SUBCENTERN Government -

Police Station List
PS CODE POLOCE STATION NAME ADDRESS DIST CODEDIST NAME TK CODETALUKA NAME 1 YESHWANTHPUR PS BANGALORE 20 BANGALORE 1 Bangalore North 2 JALAHALLI PS BANGALORE 20 BANGALORE 1 Bangalore North 3 RMC YARD PS BANGALORE 20 BANGALORE 1 Bangalore North 4 PEENYA PS BANGALORE 20 BANGALORE 1 Bangalore North 5 GANGAMMAGUDI PS BANGALORE 20 BANGALORE 1 Bangalore North 6 SOLADEVANAHALLI PS BANGALORE 20 BANGALORE 1 Bangalore North 7 MALLESWARAM PS BANGALORE 20 BANGALORE 1 Bangalore North 8 SRIRAMPURAM PS BANGALORE 20 BANGALORE 1 Bangalore North 9 RAJAJINAGAR PS BANGALORE 20 BANGALORE 1 Bangalore North 10 MAHALAXMILAYOUT PS BANGALORE 20 BANGALORE 1 Bangalore North 11 SUBRAMANYANAGAR PS BANGALORE 20 BANGALORE 1 Bangalore North 12 RAJAGOPALNAGAR PS BANGALORE 20 BANGALORE 1 Bangalore North 13 NANDINI LAYOUT PS BANGALORE 20 BANGALORE 1 Bangalore North 14 J C NAGAR PS BANGALORE 20 BANGALORE 1 Bangalore North 15 HEBBAL PS BANGALORE 20 BANGALORE 1 Bangalore North 16 R T NAGAR PS BANGALORE 20 BANGALORE 1 Bangalore North 17 YELAHANKA PS BANGALORE 20 BANGALORE 1 Bangalore North 18 VIDYARANYAPURA PS BANGALORE 20 BANGALORE 1 Bangalore North 19 SANJAYNAGAR PS BANGALORE 20 BANGALORE 1 Bangalore North 20 YELAHANKA NEWTOWN PS BANGALORE 20 BANGALORE 1 Bangalore North 21 CENTRAL PS BANGALORE 20 BANGALORE 2 Bangalore South 22 CHAMARAJPET PS BANGALORE 20 BANGALORE 2 Bangalore South 23 VICTORIA HOSPITAL PS BANGALORE 20 BANGALORE 2 Bangalore South 24 SHANKARPURA PS BANGALORE 20 BANGALORE 2 Bangalore South 25 RPF MANDYA MANDYA 22 MANDYA 5 Mandya 26 HANUMANTHANAGAR PS BANGALORE -

Karnataka Circle Cycle III Vide Notification R&E/2-94/GDS ONLINE CYCLE-III/2020 DATED at BENGALURU-560001, the 21-12-2020
Selection list of Gramin Dak Sevak for Karnataka circle Cycle III vide Notification R&E/2-94/GDS ONLINE CYCLE-III/2020 DATED AT BENGALURU-560001, THE 21-12-2020 S.No Division HO Name SO Name BO Name Post Name Cate No Registration Selected Candidate gory of Number with Percentage Post s 1 Bangalore Bangalore ARABIC ARABIC GDS ABPM/ EWS 1 DR1786DA234B73 MONU KUMAR- East GPO COLLEGE COLLEGE Dak Sevak (95)-UR-EWS 2 Bangalore Bangalore ARABIC ARABIC GDS ABPM/ OBC 1 DR3F414F94DC77 MEGHANA M- East GPO COLLEGE COLLEGE Dak Sevak (95.84)-OBC 3 Bangalore Bangalore ARABIC ARABIC GDS ABPM/ ST 1 DR774D4834C4BA HARSHA H M- East GPO COLLEGE COLLEGE Dak Sevak (93.12)-ST 4 Bangalore Bangalore Dr. Dr. GDS ABPM/ ST 1 DR8DDF4C1EB635 PRABHU- (95.84)- East GPO Shivarama Shivarama Dak Sevak ST Karanth Karanth Nagar S.O Nagar S.O 5 Bangalore Bangalore Dr. Dr. GDS ABPM/ UR 2 DR5E174CAFDDE SACHIN ADIVEPPA East GPO Shivarama Shivarama Dak Sevak F HAROGOPPA- Karanth Karanth (94.08)-UR Nagar S.O Nagar S.O 6 Bangalore Bangalore Dr. Dr. GDS ABPM/ UR 2 DR849944F54529 SHANTHKUMAR B- East GPO Shivarama Shivarama Dak Sevak (94.08)-UR Karanth Karanth Nagar S.O Nagar S.O 7 Bangalore Bangalore H.K.P. Road H.K.P. Road GDS ABPM/ SC 1 DR873E54C26615 AJAY- (95)-SC East GPO S.O S.O Dak Sevak 8 Bangalore Bangalore HORAMAVU HORAMAVU GDS ABPM/ SC 1 DR23DCD1262A44 KRISHNA POL- East GPO Dak Sevak (93.92)-SC 9 Bangalore Bangalore Kalyananagar Banaswadi GDS ABPM/ OBC 1 DR58C945D22D77 JAYANTH H S- East GPO S.O S.O Dak Sevak (97.6)-OBC 10 Bangalore Bangalore Kalyananagar Kalyananagar GDS ABPM/ OBC 1 DR83E4F8781D9A MAMATHA S- East GPO S.O S.O Dak Sevak (96.32)-OBC 11 Bangalore Bangalore Kalyananagar Kalyananagar GDS ABPM/ UR 1 DR26EE624216A1 DHANYATA S East GPO S.O S.O Dak Sevak NAYAK- (95.8)-UR 12 Bangalore Bangalore St. -

Prl District and Sessions Judge, Gadag RAJASHEKAR VENKANGOUDA PATIL Prl.District and Sessions Judge Cause List Date: 19-12-2020
Prl District and Sessions Judge, Gadag RAJASHEKAR VENKANGOUDA PATIL Prl.District and Sessions Judge Cause List Date: 19-12-2020 Sr. No. Case Number Timing/Next Date Party Name Advocate 02-45 to 05-45 PM 1 EX 279/2017 M/s Sundaram Finance Limitied, S.P.Patil (Refer to Lok Adalath) Chennai, Repted by Nagaraj Palankar Vs Nagaraj Ningappa Ragati 2 EX 292/2017 M/s Indusind Bank Ltd., Hubli S S Kori (Refer to Lok Adalath) Rept. by its GPA Holder, Shivashankaragouda Parwathagouda Patil Vs Vittal Premanathsa Bhandage 3 EX 36/2018 HDB Financial Services Ltd., M S Halakeri (Refer to Lok Adalath) Hubballi Rept. by its Representative Officer Vishalkumar B Doddagoudar Vs Shafasil Mohammedhafeez Khairati Mohammed Hafeez Khairathi 4 EX 37/2018 HDB Financial Services Ltd., M S Halakeri (Refer to Lok Adalath) Hubballi Rept. by its Representative Officer Vishalkumar B Doddagoudar Vs Mudassaranazar M Khairati Mohammed Hafeez Khairathi 5 EX 66/2018 M/s Sriram Transport Finance K R Naikar (Refer to Lok Adalath) Company Ltd., Gadag Rept. by its PA Holder Shankar Basappa Sutagatti Vs Raghvendra B Sathannavar 6 EX 76/2018 M/s Sriram Transport Finance K R Naikar (Refer to Lok Adalath) Company Ltd., Gadag Rept. by its PA Holder Shankar Basappa Sutagatti Vs Bharamappa Ningappa Hittalamani 7 EX 127/2018 M/s Sriram Transport Finance S B Challamarad (Refer to Lok Adalath) Company Ltd., Gadag Rept. by its PA Holder Shankar Basappa Sutagatti Vs Mohammadrafiq Mohamadhussain Dukandhur 1/5 Prl District and Sessions Judge, Gadag RAJASHEKAR VENKANGOUDA PATIL Prl.District and Sessions Judge Cause List Date: 19-12-2020 Sr.