Drug-Eluting Coronary Stent System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fig. L COMPOSITIONS and METHODS to INHIBIT STEM CELL and PROGENITOR CELL BINDING to LYMPHOID TISSUE and for REGENERATING GERMINAL CENTERS in LYMPHATIC TISSUES
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date Χ 23 February 2012 (23.02.2012) WO 2U12/U24519ft ft A2 (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, A61K 31/00 (2006.01) CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, PCT/US201 1/048297 KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (22) International Filing Date: ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, 18 August 201 1 (18.08.201 1) NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (25) Filing Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (26) Publication Language: English ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/374,943 18 August 2010 (18.08.2010) US kind of regional protection available): ARIPO (BW, GH, 61/441,485 10 February 201 1 (10.02.201 1) US GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, 61/449,372 4 March 201 1 (04.03.201 1) US ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (72) Inventor; and EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, ΓΓ, LT, LU, (71) Applicant : DEISHER, Theresa [US/US]; 1420 Fifth LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, Avenue, Seattle, WA 98101 (US). -

Coronary Artery Perforation
Published online: 2019-09-20 THIEME 110 InterventionalCoronary Artery Rounds Perforation Deb et al. Coronary Artery Perforation Tripti Deb1 Jyotsna Maddury2 Prasant Kr. Sahoo3 1Department of Interventional Cardiology, Apollo Hospitals, Address for correspondence Jyotsna Maddury, MD, DM, FACC, Hyderabad, Telangana, India Department of Cardiology, Nizam’s Institute of Medical Sciences 2Department of Cardiology, Nizam’s Institute of Medical Sciences (NIMS), Punjagutta, Hyderabad, Telangana 500082, India (NIMS), Punjagutta, Hyderabad, India (e-mail: [email protected]). 3Department of Interventional Cardiology, Apollo Hospitals, Bhubaneswar, Odisha, India Ind J Car Dis Wom 2019;4:110–120 Abstract Percutaneous coronary intervention (PCI) is considered as the standard treatment of obstructive coronary artery disease in indicated patients. Even though PCI gives symp- Keywords tomatic angina improvement, but associated with serious complications like coronary ► coronary artery artery perforation (CAP), the incidence is quite low. With the more complex lesions for perforation successful angioplasty, different devices are required, which in turn increase the inci- ► percutaneous coro- dence of CAP in these patients. Here we review the classification, incidence, pathogen- nary intervention esis, clinical sequela, risk factors, predictors, and management of CAP in the current ► coronary artery era due to PCI. perforation ► management of coro- nary perfusion Introduction Consequences of Coronary Artery Perforation Percutaneous coronary intervention (PCI) for obstructive coronary artery diseases is accepted and has standardized Consequences of CAP depend on the location and severity. procedure with minimal complication rates, including iat- Location wise, if CAP occurs to the right or left ventricle, if not rogenic coronary artery perforation (CAP). Although angio- massive, then usually no immediate clinical consequences graphically significant coronary artery dissection is known occur. -

3Rd Quarter 2001 Bulletin
In This Issue... Promoting Colorectal Cancer Screening Important Information and Documentaion on Promoting the Prevention of Colorectal Cancer ....................................................................................................... 9 Intestinal and Multi-Visceral Transplantation Coverage Guidelines and Requirements for Approval of Transplantation Facilities12 Expanded Coverage of Positron Emission Tomography Scans New HCPCS Codes and Coverage Guidelines Effective July 1, 2001 ..................... 14 Skilled Nursing Facility Consolidated Billing Clarification on HCPCS Coding Update and Part B Fee Schedule Services .......... 22 Final Medical Review Policies 29540, 33282, 67221, 70450, 76090, 76092, 82947, 86353, 93922, C1300, C1305, J0207, and J9293 ......................................................................................... 31 Outpatient Prospective Payment System Bulletin Devices Eligible for Transitional Pass-Through Payments, New Categories and Crosswalk C-codes to Be Used in Coding Devices Eligible for Transitional Pass-Through Payments ............................................................................................ 68 Features From the Medical Director 3 he Medicare A Bulletin Administrative 4 Tshould be shared with all General Information 5 health care practitioners and managerial members of the General Coverage 12 provider/supplier staff. Hospital Services 17 Publications issued after End Stage Renal Disease 19 October 1, 1997, are available at no-cost from our provider Skilled Nursing Facility -

ICD~10~PCS Complete Code Set Procedural Coding System Sample
ICD~10~PCS Complete Code Set Procedural Coding System Sample Table.of.Contents Preface....................................................................................00 Mouth and Throat ............................................................................. 00 Introducton...........................................................................00 Gastrointestinal System .................................................................. 00 Hepatobiliary System and Pancreas ........................................... 00 What is ICD-10-PCS? ........................................................................ 00 Endocrine System ............................................................................. 00 ICD-10-PCS Code Structure ........................................................... 00 Skin and Breast .................................................................................. 00 ICD-10-PCS Design ........................................................................... 00 Subcutaneous Tissue and Fascia ................................................. 00 ICD-10-PCS Additional Characteristics ...................................... 00 Muscles ................................................................................................. 00 ICD-10-PCS Applications ................................................................ 00 Tendons ................................................................................................ 00 Understandng.Root.Operatons..........................................00 -

DRUG and MEDICAL DEVICE HIGHLIGHTS Helping You Maintain and Improve Your Health 2019
DRUG AND MEDICAL DEVICE HIGHLIGHTS Helping you maintain and improve your health 2019 DRUG AND MEDICAL DEVICE HIGHLIGHTS 2019 Helping you maintain and improve your health Learn about the new drugs and medical devices that Health Canada approved for sale in Canada, the information we published about potential safety issues, and our other accomplishments in 2019. Health Canada is the federal department responsible for helping the people of Canada maintain and improve their health. Health Canada is committed to improving the lives of all of Canada’s people and to making this country’s population among the healthiest in the world as measured by longevity, lifestyle and effective use of the public health care system. Également disponible en français sous le titre : Préserver et améliorer votre santé : Faits saillants sur les médicaments et les instruments médicaux 2019 To obtain additional information, please contact: Health Canada Address Locator 0900C2 Ottawa, ON K1A 0K9 Tel.: 613-957-2991 Toll free: 1-866-225-0709 Fax: 613-941-5366 TTY: 1-800-465-7735 E-mail: [email protected] © Her Majesty the Queen in Right of Canada, as represented by the Minister of Health, 2020 Publication date: May 2020 This publication may be reproduced for personal or internal use only without permission provided the source is fully acknowledged. Cat.: H161-11E-PDF ISSN: 2562-9816 Pub.: 190484 CONTENTS WELCOME TO OUR 2019 HIGHLIGHTS REPORT ..........................................................................................1 MESSAGE FROM THE CHIEF MEDICAL -

Two Cases of Immediate Stent Fracture After Zotarolimus-Eluting
Case Report http://dx.doi.org/10.4070/kcj.2015.45.1.67 Print ISSN 1738-5520 • On-line ISSN 1738-5555 Korean Circulation Journal Two Cases of Immediate Stent Fracture after Zotarolimus-Eluting Stent Implantation Pil Hyung Lee, MD, Seung-Whan Lee, MD, Jong-Young Lee, MD, Young-Hak Kim, MD, Cheol Whan Lee, MD, Duk-Woo Park, MD, Seong-Wook Park, MD, and Seung-Jung Park, MD Department of Cardiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea Drug-eluting stent (DES) implantation is currently the standard treatment for various types of coronary artery disease. However, previous reports indicate that stent fractures, which usually occur after a period of time from the initial DES implantation, have increased during the DES era; stent fractures can contribute to unfavorable events such as in-stent restenosis and stent thrombosis. In our present report, we describe two cases of zotarolimus-eluting stent fracture: one that was detected six hours after implementation, and the other case that was detected immediately after deployment. Both anatomical and technical risk factors contributed to these unusual cases of imme- diate stent fracture. (Korean Circ J 2015;45(1):67-70) KEY WORDS: Drug-eluting stents; Percutaneous coronary intervention; Complications. Introduction eluting stent (ZES). Stent fractures have recently become an important concern in Cases the medical community due to their potential association with se- rious conditions such as in-stent restenosis (ISR) and stent throm- Case 1 bosis after drug-eluting stent (DES) implantation.1) Most currently A 62-year-old man, who had undergone cardiac transplantation reported stent fractures are found in lesions implanted with siroli- for advanced heart failure, was referred to our catheterization labo- mus-eluting stents (SES), likely due to the inherent platform material ratory for a regular surveillance coronary angiogram and concomi- and design of such stents.2) Furthermore, while the exact timing of tant intravascular ultrasound (IVUS) imaging. -

Ventricular Long Axis Function: Amplitudes and Timings
Umeå University Medical Dissertations New Series No 895 - ISSN 0346-6612 _______________________________________________________________ From the Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden Ventricular Long Axis Function: Amplitudes and Timings Echocardiographic Studies in Health and Disease Frederick Bukachi Umeå 2004 © Copyright: Frederick Bukachi ISBN 91-7305-661-8 Printed in Sweden by Solfjädern offset AB Umeå, 2004 To my family Frida, Anthony, Arnold and Maria Time brings wisdom to the mind and healing to the heart. Anonymous Table of contents Table of contents................................................................................................................. 1 Abstract ................ ............................................................................................................. 3 List of original studies ........................................................................................................4 Abbreviations...................................................................................................................... 5 1. Introduction............................................................................................................. 6 2. Ventricular long axis ............................................................................................... 6 2.1. General overview and historical perspective................................................ 6 2.2. Anatomy of long axis .................................................................................. -

TAXUS™ Express 2 ™ Paclitaxel-Eluting
TAXU S ® Express 2® Paclitaxel-Eluting Coronary Stent System Patient Information Guide Table of Contents Notes Coronary Artery Disease . 2 Who is at Risk? . .3 Diagnosis of Coronary Artery Disease . 3 Treatment of Coronary Artery Disease . 3 Angioplasty . 4 Coronary Artery Stents . 4 Restenosis . 5 Your Drug-Eluting Stent, the TAXUS ® Express 2® Paclitaxel-Eluting Coronary Stent System . 7 Drug-Eluting Stents . 7 The Express ® Stent Platform for the TAXUS ® Express ® Stent . 7 The Polymer Coating on the TAXUS Express Stent . 7 The Drug that is Released from the TAXUS Express Stent . 8 When should the TAXUS Express Stent NOT be Used . 8 What are the Risks & Potential Benefits of Treatment with the TAXUS Express Stent? . 9 Alternative Practices and Procedures . 11 The Angioplasty Procedure . 12 Preparation for the Procedure . 12 Angioplasty and Stent Placement Procedure . 12 Post-Treatment . 13 After the Procedure . 13 Activity . 14 Medications . 14 Follow-Up Examinations . 15 Magnetic Resonance Imaging (MRI) . 15 Frequently Asked Questions . 16 Glossary . 17 Patient Information Card . Inside Back Cover 1 Notes Coronary Artery Disease Coronary Artery Disease (CAD) is usually caused by atherosclerosis, and affects the coronary arteries that surround the heart. These coronary arteries supply blood with oxygen and other nutrients to the heart muscle to make it function properly. CAD occurs when the inner walls of the coronary arteries thicken due to a buildup of cholesterol, fatty deposits, calcium, and other elements. This material is known as plaque. As plaque develops, the vessel narrows. When the vessel narrows (for example with physical exertion or mental stress), Aorta blood flow through the vessel is reduced so less oxygen and Right Left other nutrients reach Coronary Coronary the heart muscle. -

Related Kinases (VRK) Bound to Small-Molecule Inhibitors Identifies Different P-Loop Conformations
Structural characterization of human Vaccinia- Related Kinases (VRK) bound to small-molecule inhibitors identifies different P-loop conformations Rafael M. Couñago1,2*, Charles K. Allerston3, Pavel Savitsky3, Hatylas Azevedo4, Paulo H Godoi1,5, Carrow I. Wells6, Alessandra Mascarello4, Fernando H. de Souza Gama4, Katlin B. Massirer1,2, William J. Zuercher6, Cristiano R.W. Guimarães4 and Opher Gileadi1,3 1. Structural Genomics Consortium, Universidade Estadual de Campinas — UNICAMP, Campinas, SP, Brazil. 2. Centro de Biologia Molecular e Engenharia Genética, Universidade Estadual de Campinas, Campinas, SP, Brazil. 3. Structural Genomics Consortium and Target Discovery Institute, Nuffield Department of Clinical Medicine, University of Oxford, UK. 4. Aché Laboratórios Farmacêuticos SA, Guarulhos, SP, Brazil. 5. Department of Biochemistry and Tissue Biology, Institute of Biology, State University of Campinas, Campinas, Brazil. 6. Structural Genomics Consortium, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, NC, USA. *Correspondence to Rafael M. Couñago: [email protected] (RMC) Supplementary information 1 SUPPLEMENTARY METHODS PKIS results analyses - hierarchical cluster analysis (HCL) A hierarchical clustering (HCL) analysis was performed to group kinases based on their inhibition patterns across the compounds. The average distance clustering method was employed, using sample tree selection and sample leaf order optimization. The distance metric used was the Pearson correlation and the HCL analysis was performed in the TmeV software 1. SUPPLEMENTARY REFERENCES 1 Saeed, A. I. et al. TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34, 374-378, (2003). SUPPLEMENTARY FIGURES LEGENDS Supplementary Figure S1: Hierarchical clustering analysis of PKIS data. Hierarchical clustering analysis of PKIS data. -

Clinical Appropriateness Guidelines: Percutaneous Coronary Intervention
Clinical Appropriateness Guidelines: Percutaneous Coronary Intervention Appropriate Use Criteria Effective Date: January 2, 2018 Proprietary Date of Origin: 08/27/2015 Last revised: 08/01/2017 Last reviewed: 09/07/2017 8600 W Bryn Mawr Avenue South Tower - Suite 800 Chicago, IL 60631 P. 773.864.4600 Copyright © 2018. AIM Specialty Health. All Rights Reserved www.aimspecialtyhealth.com Table of Contents Description and Application of the Guidelines ........................................................................3 Percutaneous Coronary Intervention .......................................................................................4 Table of Contents | Copyright © 2018. AIM Specialty Health. All Rights Reserved. 2 Description and Application of the Guidelines AIM’s Clinical Appropriateness Guidelines (hereinafter “AIM’s Clinical Appropriateness Guidelines” or the “Guidelines”) are designed to assist providers in making the most appropriate treatment decision for a specific clinical condition for an individual. As used by AIM, the Guidelines establish objective and evidence-based, where possible, criteria for medical necessity determinations. In the process, multiple functions are accomplished: ● To establish criteria for when services are medically necessary ● To assist the practitioner as an educational tool ● To encourage standardization of medical practice patterns ● To curtail the performance of inappropriate and/or duplicate services ● To advocate for patient safety concerns ● To enhance the quality of healthcare ● To promote the most efficient and cost-effective use of services AIM’s guideline development process complies with applicable accreditation standards, including the requirement that the Guidelines be developed with involvement from appropriate providers with current clinical expertise relevant to the Guidelines under review and be based on the most up to date clinical principles and best practices. Relevant citations are included in the “References” section attached to each Guideline. -

Everolimus Eluting Coronary Stent System
Everolimus Eluting Coronary Stent System Patient Information Guide Table of Contents Coronary Artery Disease (CAD) ........................5 Your Drug-Eluting Stent Procedure ................19 Your Heart ........................................................5 How Do I Prepare for My Procedure? ...........19 What is CAD? ..................................................5 Your Drug-Eluting Stent Placement What are the Symptoms of CAD? ...................5 Procedure ......................................................19 What are the Risk Factors of CAD? ................6 Immediately after Procedure .........................22 How Can My Doctor Tell if I Have CAD? .........7 Take All Medications as Instructed ................22 Follow-up Care ..............................................23 Your Treatment Options .....................................8 Keep Your ID Card Handy .............................23 Surgery .............................................................9 Angioplasty ......................................................9 Preventing CAD ................................................24 Coronary Artery Stents ..................................10 Frequently Asked Questions ...........................25 Drug-Eluting Stents (DES) ...............................11 Definition of Medical Terms ............................26 XIENCE Family of Coronary Stents ................12 Contraindications ...........................................13 Potential Adverse Events Associated with the XIENCE Family of Coronary Stents ..........13 -
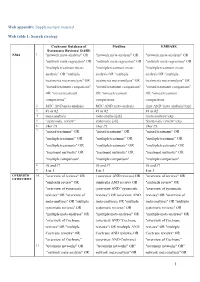
1 Web Appendix: Supplementary Material Web Table 1
Web appendix: Supplementary material Web table 1: Search strategy Cochrane Database of Medline EMBASE Systematic Reviews/ DARE NMA 1 "network meta-analysis" OR "network meta-analysis" OR "network meta-analysis" OR "network meta-regression" OR "network meta-regression" OR "network meta-regression" OR "multiple treatment meta- "multiple treatment meta- "multiple treatment meta- analysis” OR "multiple analysis OR "multiple analysis OR "multiple treatments meta-analysis" OR treatments meta-analysis" OR treatments meta-analysis" OR "mixed treatment comparison" "mixed treatment comparison" "mixed treatment comparison" OR "mixed treatment OR "mixed treatment OR "mixed treatment comparisons” comparisons comparisons 2 MTC AND meta-analysis MTC AND meta-analysis (mtc AND 'meta analysis'/exp) 3 #1 or #2 #1 or #2 #1 or #2 4 meta-analysis meta-analysis[sb] 'meta analysis'/exp 5 “systematic review” systematic [sb] 'Systematic review '/exp 6 #4or #5 #4or #5 #4or #5 7 "mixed treatment" OR "mixed treatment" OR "mixed treatment" OR "multiple treatment" OR "multiple treatment" OR "multiple treatment" OR "multiple treatments" OR "multiple treatments" OR "multiple treatments" OR "treatment networks" OR "treatment networks" OR "treatment networks" OR "multiple comparison" "multiple comparison" "multiple comparison" 8 #6 and #7 #6 and #7 #6 and #7 9 8 or 3 8 or 3 8 or 3 OVERVIEW 10 "overview of reviews" OR ( overview AND reviews) OR "overview of reviews" OR OF REVIEWS "umbrella review" OR (umbrella AND review) OR "umbrella review" OR "overview of systematic