Invertebrate Colonization of Leaves and Roots Within Sediments of Intermittent Coastal Plain Streams Across Hydrologic Phases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHIRONOMUS Newsletter on Chironomidae Research
CHIRONOMUS Newsletter on Chironomidae Research No. 25 ISSN 0172-1941 (printed) 1891-5426 (online) November 2012 CONTENTS Editorial: Inventories - What are they good for? 3 Dr. William P. Coffman: Celebrating 50 years of research on Chironomidae 4 Dear Sepp! 9 Dr. Marta Margreiter-Kownacka 14 Current Research Sharma, S. et al. Chironomidae (Diptera) in the Himalayan Lakes - A study of sub- fossil assemblages in the sediments of two high altitude lakes from Nepal 15 Krosch, M. et al. Non-destructive DNA extraction from Chironomidae, including fragile pupal exuviae, extends analysable collections and enhances vouchering 22 Martin, J. Kiefferulus barbitarsis (Kieffer, 1911) and Kiefferulus tainanus (Kieffer, 1912) are distinct species 28 Short Communications An easy to make and simple designed rearing apparatus for Chironomidae 33 Some proposed emendations to larval morphology terminology 35 Chironomids in Quaternary permafrost deposits in the Siberian Arctic 39 New books, resources and announcements 43 Finnish Chironomidae 47 Chironomini indet. (Paratendipes?) from La Selva Biological Station, Costa Rica. Photo by Carlos de la Rosa. CHIRONOMUS Newsletter on Chironomidae Research Editors Torbjørn EKREM, Museum of Natural History and Archaeology, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway Peter H. LANGTON, 16, Irish Society Court, Coleraine, Co. Londonderry, Northern Ireland BT52 1GX The CHIRONOMUS Newsletter on Chironomidae Research is devoted to all aspects of chironomid research and aims to be an updated news bulletin for the Chironomidae research community. The newsletter is published yearly in October/November, is open access, and can be downloaded free from this website: http:// www.ntnu.no/ojs/index.php/chironomus. Publisher is the Museum of Natural History and Archaeology at the Norwegian University of Science and Technology in Trondheim, Norway. -

Zootaxa, Japanese Pseudosmittia Edwards (Diptera: Chironomidae)
Zootaxa 1198: 21–51 (2006) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 1198 Copyright © 2006 Magnolia Press ISSN 1175-5334 (online edition) Japanese Pseudosmittia Edwards (Diptera: Chironomidae) OLE A. SÆTHER The Natural History Collections, Bergen Museum, University of Bergen, N-5020 Bergen, Norway. E-mail: [email protected] Abstract The types of species previously placed in Pseudosmittia Edwards and some related genera in the Sasa collection at The National Museum of Sciences, Tokyo, Japan, have been examined. Twenty- four new synonyms are given: Pseudosmittia ogasatridecima Sasa et Suzuki, 1997a is a synonym of P. bifurcata (Tokunaga, 1936); P. jintuvicesima Sasa, 1996, and P. seiryupequea Sasa, Suzuki et Sakai, 1998 of P. danconai (Marcuzzi, 1947); P. mongolzeaea Sasa et Suzuki, 1997b of P. f orc ipa ta (Goetghebuer, 1921); P. hachijotertia Sasa, 1994 of P. holsata Thienemann et Strenzke, 1940; P. itachibifurca Sasa et Kawai, 1987, P. furudobifurca Sasa et Arakawa, 1994, P. hibaribifurca Sasa, 1993, and P. (Nikismittia) shofukuundecima Sasa, 1998 of P. mathildae Albu, 1968; P. yakymenea Sasa et Suzuki, 2000a, and P. yakyneoa Sasa et Suzuki, 2000a of P. nishiharaensis Sasa et Hasegawa, 1988; P. kurobeokasia Sasa et Okazawa, 1992a, P. togarisea Sasa et Okazawa, 1992b, P. hachijosecunda Sasa, 1994, P. to ya m a re s e a Sasa, 1996, P. yakyopea Sasa et Suzuki, 2000a, P. yakypequea Sasa et Suzuki, 2000a, Parakiefferiella hidakagehea Sasa et Suzuki, 2000b, and Parakiefferiella hidakaheia Sasa et Suzuki, 2000b of Pseudosmittia oxoniana (Edwards, 1922); P. famikelea Sasa, 1996a of P. tokaraneoa Sasa et Suzuki, 1995; P. -

Chironomidae Hirschkopf
Literatur Chironomidae Gesäuse U.A. zur Bestimmung und Ermittlung der Autökologie herangezogene Literatur: Albu, P. (1972): Două specii de Chironomide noi pentru ştiinţă în masivul Retezat.- St. şi Cerc. Biol., Seria Zoologie, 24: 15-20. Andersen, T.; Mendes, H.F. (2002): Neotropical and Mexican Mesosmittia Brundin, with the description of four new species (Insecta, Diptera, Chironomidae).- Spixiana, 25(2): 141-155. Andersen, T.; Sæther, O.A. (1993): Lerheimia, a new genus of Orthocladiinae from Africa (Diptera: Chironomidae).- Spixiana, 16: 105-112. Andersen, T.; Sæther, O.A.; Mendes, H.F. (2010): Neotropical Allocladius Kieffer, 1913 and Pseudosmittia Edwards, 1932 (Diptera: Chironomidae).- Zootaxa, 2472: 1-77. Baranov, V.A. (2011): New and rare species of Orthocladiinae (Diptera, Chironomidae) from the Crimea, Ukraine.- Vestnik zoologii, 45(5): 405-410. Boggero, A.; Zaupa, S.; Rossaro, B. (2014): Pseudosmittia fabioi sp. n., a new species from Sardinia (Diptera: Chironomidae, Orthocladiinae).- Journal of Entomological and Acarological Research, [S.l.],46(1): 1-5. Brundin, L. (1947): Zur Kenntnis der schwedischen Chironomiden.- Arkiv för Zoologi, 39 A(3): 1- 95. Brundin, L. (1956): Zur Systematik der Orthocladiinae (Dipt. Chironomidae).- Rep. Inst. Freshwat. Drottningholm 37: 5-185. Casas, J.J.; Laville, H. (1990): Micropsectra seguyi, n. sp. du groupe attenuata Reiss (Diptera: Chironomidae) de la Sierra Nevada (Espagne).- Annls Soc. ent. Fr. (N.S.), 26(3): 421-425. Caspers, N. (1983): Chironomiden-Emergenz zweier Lunzer Bäche, 1972.- Arch. Hydrobiol. Suppl. 65: 484-549. Caspers, N. (1987): Chaetocladius insolitus sp. n. (Diptera: Chironomidae) from Lunz, Austria. In: Saether, O.A. (Ed.): A conspectus of contemporary studies in Chironomidae (Diptera). -

Checklist of the Family Chironomidae (Diptera) of Finland
A peer-reviewed open-access journal ZooKeys 441: 63–90 (2014)Checklist of the family Chironomidae (Diptera) of Finland 63 doi: 10.3897/zookeys.441.7461 CHECKLIST www.zookeys.org Launched to accelerate biodiversity research Checklist of the family Chironomidae (Diptera) of Finland Lauri Paasivirta1 1 Ruuhikoskenkatu 17 B 5, FI-24240 Salo, Finland Corresponding author: Lauri Paasivirta ([email protected]) Academic editor: J. Kahanpää | Received 10 March 2014 | Accepted 26 August 2014 | Published 19 September 2014 http://zoobank.org/F3343ED1-AE2C-43B4-9BA1-029B5EC32763 Citation: Paasivirta L (2014) Checklist of the family Chironomidae (Diptera) of Finland. In: Kahanpää J, Salmela J (Eds) Checklist of the Diptera of Finland. ZooKeys 441: 63–90. doi: 10.3897/zookeys.441.7461 Abstract A checklist of the family Chironomidae (Diptera) recorded from Finland is presented. Keywords Finland, Chironomidae, species list, biodiversity, faunistics Introduction There are supposedly at least 15 000 species of chironomid midges in the world (Armitage et al. 1995, but see Pape et al. 2011) making it the largest family among the aquatic insects. The European chironomid fauna consists of 1262 species (Sæther and Spies 2013). In Finland, 780 species can be found, of which 37 are still undescribed (Paasivirta 2012). The species checklist written by B. Lindeberg on 23.10.1979 (Hackman 1980) included 409 chironomid species. Twenty of those species have been removed from the checklist due to various reasons. The total number of species increased in the 1980s to 570, mainly due to the identification work by me and J. Tuiskunen (Bergman and Jansson 1983, Tuiskunen and Lindeberg 1986). -

Table of Contents 2
Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT) List of Freshwater Macroinvertebrate Taxa from California and Adjacent States including Standard Taxonomic Effort Levels 1 March 2011 Austin Brady Richards and D. Christopher Rogers Table of Contents 2 1.0 Introduction 4 1.1 Acknowledgments 5 2.0 Standard Taxonomic Effort 5 2.1 Rules for Developing a Standard Taxonomic Effort Document 5 2.2 Changes from the Previous Version 6 2.3 The SAFIT Standard Taxonomic List 6 3.0 Methods and Materials 7 3.1 Habitat information 7 3.2 Geographic Scope 7 3.3 Abbreviations used in the STE List 8 3.4 Life Stage Terminology 8 4.0 Rare, Threatened and Endangered Species 8 5.0 Literature Cited 9 Appendix I. The SAFIT Standard Taxonomic Effort List 10 Phylum Silicea 11 Phylum Cnidaria 12 Phylum Platyhelminthes 14 Phylum Nemertea 15 Phylum Nemata 16 Phylum Nematomorpha 17 Phylum Entoprocta 18 Phylum Ectoprocta 19 Phylum Mollusca 20 Phylum Annelida 32 Class Hirudinea Class Branchiobdella Class Polychaeta Class Oligochaeta Phylum Arthropoda Subphylum Chelicerata, Subclass Acari 35 Subphylum Crustacea 47 Subphylum Hexapoda Class Collembola 69 Class Insecta Order Ephemeroptera 71 Order Odonata 95 Order Plecoptera 112 Order Hemiptera 126 Order Megaloptera 139 Order Neuroptera 141 Order Trichoptera 143 Order Lepidoptera 165 2 Order Coleoptera 167 Order Diptera 219 3 1.0 Introduction The Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT) is charged through its charter to develop standardized levels for the taxonomic identification of aquatic macroinvertebrates in support of bioassessment. This document defines the standard levels of taxonomic effort (STE) for bioassessment data compatible with the Surface Water Ambient Monitoring Program (SWAMP) bioassessment protocols (Ode, 2007) or similar procedures. -

DNA Barcoding
Full-time PhD studies of Ecology and Environmental Protection Piotr Gadawski Species diversity and origin of non-biting midges (Chironomidae) from a geologically young lake PhD Thesis and its old spring system Performed in Department of Invertebrate Zoology and Hydrobiology in Institute of Ecology and Environmental Protection Różnorodność gatunkowa i pochodzenie fauny Supervisor: ochotkowatych (Chironomidae) z geologicznie Prof. dr hab. Michał Grabowski młodego jeziora i starego systemu źródlisk Auxiliary supervisor: Dr. Matteo Montagna, Assoc. Prof. Łódź, 2020 Łódź, 2020 Table of contents Acknowledgements ..........................................................................................................3 Summary ...........................................................................................................................4 General introduction .........................................................................................................6 Skadar Lake ...................................................................................................................7 Chironomidae ..............................................................................................................10 Species concept and integrative taxonomy .................................................................12 DNA barcoding ...........................................................................................................14 Chapter I. First insight into the diversity and ecology of non-biting midges (Diptera: Chironomidae) -
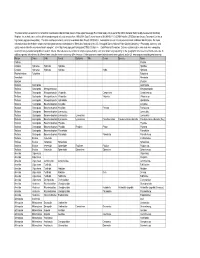
This Table Contains a Taxonomic List of Benthic Invertebrates Collected from Streams in the Upper Mississippi River Basin Study
This table contains a taxonomic list of benthic invertebrates collected from streams in the Upper Mississippi River Basin study unit as part of the USGS National Water Quality Assessemnt (NAWQA) Program. Invertebrates were collected from woody snags in selected streams from 1996-2004. Data Retreival occurred 26-JAN-06 11.10.25 AM from the USGS data warehouse (Taxonomic List Invert http://water.usgs.gov/nawqa/data). The data warehouse currently contains invertebrate data through 09/30/2002. Invertebrate taxa can include provisional and conditional identifications. For more information about invertebrate sample processing and taxonomic standards see, "Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory -- Processing, taxonomy, and quality control of benthic macroinvertebrate samples", at << http://nwql.usgs.gov/Public/pubs/OFR00-212.html >>. Data Retrieval Precaution: Extreme caution must be exercised when comparing taxonomic lists generated using different search criteria. This is because the number of samples represented by each taxa list will vary depending on the geographic criteria selected for the retrievals. In addition, species lists retrieved at different times using the same criteria may differ because: (1) the taxonomic nomenclature (names) were updated, and/or (2) new samples containing new taxa may Phylum Class Order Family Subfamily Tribe Genus Species Taxon Porifera Porifera Cnidaria Hydrozoa Hydroida Hydridae Hydridae Cnidaria Hydrozoa Hydroida Hydridae Hydra Hydra sp. Platyhelminthes Turbellaria Turbellaria Nematoda Nematoda Bryozoa Bryozoa Mollusca Gastropoda Gastropoda Mollusca Gastropoda Mesogastropoda Mesogastropoda Mollusca Gastropoda Mesogastropoda Viviparidae Campeloma Campeloma sp. Mollusca Gastropoda Mesogastropoda Viviparidae Viviparus Viviparus sp. Mollusca Gastropoda Mesogastropoda Hydrobiidae Hydrobiidae Mollusca Gastropoda Basommatophora Ancylidae Ancylidae Mollusca Gastropoda Basommatophora Ancylidae Ferrissia Ferrissia sp. -

Effects of Hydrological Connectivity on the Benthos of a Large River (Lower Mississippi River, USA)
University of Mississippi eGrove Electronic Theses and Dissertations Graduate School 1-1-2018 Effects of Hydrological Connectivity on the Benthos of a Large River (Lower Mississippi River, USA) Audrey B. Harrison University of Mississippi Follow this and additional works at: https://egrove.olemiss.edu/etd Part of the Biology Commons Recommended Citation Harrison, Audrey B., "Effects of Hydrological Connectivity on the Benthos of a Large River (Lower Mississippi River, USA)" (2018). Electronic Theses and Dissertations. 1352. https://egrove.olemiss.edu/etd/1352 This Dissertation is brought to you for free and open access by the Graduate School at eGrove. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of eGrove. For more information, please contact [email protected]. EFFECTS OF HYDROLOGICAL CONNECTIVITY ON THE BENTHOS OF A LARGE RIVER (LOWER MISSISSIPPI RIVER, USA) A Dissertation presented in partial fulfillment of requirements for the degree of Doctor of Philosophy in the Department of Biological Sciences The University of Mississippi by AUDREY B. HARRISON May 2018 Copyright © 2018 by Audrey B. Harrison All rights reserved. ABSTRACT The effects of hydrological connectivity between the Mississippi River main channel and adjacent secondary channel and floodplain habitats on macroinvertebrate community structure, water chemistry, and sediment makeup and chemistry are analyzed. In river-floodplain systems, connectivity between the main channel and the surrounding floodplain is critical in maintaining ecosystem processes. Floodplains comprise a variety of aquatic habitat types, including frequently connected secondary channels and oxbows, as well as rarely connected backwater lakes and pools. Herein, the effects of connectivity on riverine and floodplain biota, as well as the impacts of connectivity on the physiochemical makeup of both the water and sediments in secondary channels are examined. -

“When We Went Onto the Land, the Elders Taught Me to Not Drink from a Lake If There Were No Insects, Because That Lake Was Dead.”
“When we went onto the land, the elders taught me to not drink from a lake if there were no insects, because that lake was dead.” - Inuvialuit community member, Personal communication, 2017 THE IMPACTS OF RECENT WILDFIRES ON STREAM WATER QUALITY AND MACROINVERTEBRATE ASSEMBLAGES IN SOUTHERN NORTHWEST TERRITORIES, CANADA By Caitlin Sarah Garner, B.Sc. (Hons.) A thesis submitted to the Faculty of Graduate Studies in partial fulfillment of the requirements of the degree of Master of Sustainability Brock University St. Catharines, ON ¬2017 Caitlin S. Garner Abstract High-latitude regions are currently undergoing rapid ecosystem change due to increasing temperatures and modified precipitation regimes. Since 2012, the Northwest Territories (Canada) has been experiencing severe drought and wildfire seasons. In 2014 alone, fires within the Northwest Territories consumed over 3.4 million hectares of forested land; 1.4 times larger than the national yearly average for Canada. Wildfire is one of the most important agents influencing age structure and composition of forest stands, as such, it is a critical factor in ecosystem dynamics. The impacts of wildfire on terrestrial systems garner more attention compared to aquatic habitats. This is especially true when considering aquatic ecosystems, specifically sub-arctic streams, where the impact of fires on stream ecology and chemistry are relatively understudied. Freshwater ecosystems, such as lakes and streams, are relied upon by northern communities for their cultural significance and economic and environmental goods and services they produce, including country foods. This study examines the impact of recent wildfire on freshwater streams within the North Slave, South Slave, and Dehcho regions of the Northwest Territories (Canada) through analysis of their water chemistry and benthic macroinvertebrate assemblages. -

Microsoft Outlook
Joey Steil From: Leslie Jordan <[email protected]> Sent: Tuesday, September 25, 2018 1:13 PM To: Angela Ruberto Subject: Potential Environmental Beneficial Users of Surface Water in Your GSA Attachments: Paso Basin - County of San Luis Obispo Groundwater Sustainabilit_detail.xls; Field_Descriptions.xlsx; Freshwater_Species_Data_Sources.xls; FW_Paper_PLOSONE.pdf; FW_Paper_PLOSONE_S1.pdf; FW_Paper_PLOSONE_S2.pdf; FW_Paper_PLOSONE_S3.pdf; FW_Paper_PLOSONE_S4.pdf CALIFORNIA WATER | GROUNDWATER To: GSAs We write to provide a starting point for addressing environmental beneficial users of surface water, as required under the Sustainable Groundwater Management Act (SGMA). SGMA seeks to achieve sustainability, which is defined as the absence of several undesirable results, including “depletions of interconnected surface water that have significant and unreasonable adverse impacts on beneficial users of surface water” (Water Code §10721). The Nature Conservancy (TNC) is a science-based, nonprofit organization with a mission to conserve the lands and waters on which all life depends. Like humans, plants and animals often rely on groundwater for survival, which is why TNC helped develop, and is now helping to implement, SGMA. Earlier this year, we launched the Groundwater Resource Hub, which is an online resource intended to help make it easier and cheaper to address environmental requirements under SGMA. As a first step in addressing when depletions might have an adverse impact, The Nature Conservancy recommends identifying the beneficial users of surface water, which include environmental users. This is a critical step, as it is impossible to define “significant and unreasonable adverse impacts” without knowing what is being impacted. To make this easy, we are providing this letter and the accompanying documents as the best available science on the freshwater species within the boundary of your groundwater sustainability agency (GSA). -

Trakya Üniversitesi Fen Bilimleri Enstitüsü Binası, Balkan Yerleşkesi – 22030 Edirne / TÜRKİYE E-Mail: [email protected] Tel: +90 284 2358230 Fax: +90 284 2358237
TRAKYA UNIVERSITY JOURNAL OF NATURAL SCIENCES 20 Volume 1 Number April 2019 TUJNS TRAKYA UNIVERSITY JOURNAL OF NATURAL SCIENCES Trakya Univ J Nat Sci ISSN 2147-0294 e-ISSN 2528-9691 Trakya University Journal of Natural Sciences Volume: 20 Number: 1 April 2019 Trakya Univ J Nat Sci http://dergipark.gov.tr/trkjnat e-mail: [email protected] ISSN 2147-0294 e-ISSN 2528-9691 ISSN 2147-0294 e-ISSN 2528-9691 Trakya University Journal of Natural Sciences http://dergipark.gov.tr/trkjnat Volume 20, Number 1, April 2019 Owner On behalf of Trakya University Rectorship, Graduate School of Natural and Applied Sciences Prof. Dr. Murat YURTCAN Editor-in-Chief Doç. Dr. Kadri KIRAN Editorial Board Abdel Hameed A. AWAD National Research Center, Dokki Giza Egypt Albena LAPEVA-GJONOVA Sofia University, Sofia Bulgaria Ayşegül ÇERKEZKAYABEKİR Trakya University, Edirne Turkey (Copyeditor) Bálint MARKÓ Babeș-Bolyai University Romania Beata ZIMOWSKA University of Life Sciences, Lublin Poland Belgin SÜSLEYİCİ Marmara University, İstanbul Turkey Burak ÖTERLER Trakya University, Edirne Turkey (Design Editor) Bülent YORULMAZ Muğla Sıtkı Koçman University, Muğla Turkey Celal KARAMAN Trakya University, Edirne Turkey (Copyeditor) Cem Vural Erciyes University, Kayseri Turkey Coşkun TEZ Erciyes University, Kayseri Turkey Errol HASSAN University of Queensland, Brisbane Australia Gamze ALTINTAŞ KAZAR Trakya University, Edirne Turkey (Design Editor) Gökhan Barış ÖZDENER Boston University, Boston United States Herdem ASLAN Çanakkale Onsekiz Mart University, Çanakkale Turkey -

Assessment of the Environmental Effects Associated with Wooden Bridges Preserved with Creosote, Pentachlorophenol, Or Chromated Copper Arsenate
United States In cooperation with the Department of Agriculture Assessment of the United States Forest Service Department of Transportation Environmental Effects Forest Products Federal Laboratory Highway Associated With Wooden Administration Research Paper Bridges Preserved With FPL−RP−587 Creosote, Pentachlorophenol, or Chromated Copper Arsenate Kenneth M. Brooks Abstract However, low levels of PAHs were observed in the sedi- ments under and immediately downstream from these Timber bridges provide an economical alternative to concrete bridges. Pentachlorophenol concentrations did not approach and steel structures, particularly in rural areas with light to toxicological benchmarks. Sediment concentrations of naph- moderate vehicle traffic. Wooden components of these thalene, acenaphthylene, and phenanthrene exceeded the bridges are treated with chromated copper arsenate type C probable effect level. Metal levels at the bridges treated with (CCA), pentachlorophenol, or creosote to prolong the life of CCA were less than predicted effect levels, in spite of ques- the structure from a few years to many decades. This results tionable construction practices. Adverse biological effects in reduced transportation infrastructure costs and increased were not observed in the aquatic invertebrate community or public safety. However, the preservative used to treat the laboratory bioassays conducted on water and sediments wooden components in timber bridges is lost to the environ- sampled at each of the bridges. Results of this study reveal ment in small amounts over time. This report describes the the need to follow the construction information found in Best concentration of wood preservatives lost to adjacent envi- Management Practices for the Use of Treated Wood In ronments and the biological response to these preservatives Aquatic Environments published by Western Wood Preserv- as environmental contaminants.