Fractional Melting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geochemical Behaviour of Trace Elements During Fractional
Anais da Academia Brasileira de Ciências (2015) 87(4): 1959-1979 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201520130385 www.scielo.br/aabc Geochemical behaviour of trace elements during fractional crystallization and crustal assimilation of the felsic alkaline magmas of the state of Rio de Janeiro, Brazil AKIHISA Motoki1*, SUSANNA E. SICHEL2, THAIS Vargas1, DEAN P. MELO3 and KENJI F. Motoki2 1Departamento de Mineralogia e Petrologia Ígnea, Universidade do Estado do Rio de Janeiro, Rua São Francisco Xavier, 524, Sala A-4023, Maracanã, 20550-990 Rio de Janeiro, RJ, Brasil 2Departamento de Geologia, Universidade Federal Fluminense, Av. General Milton Cardoso, s/n, 4° Andar, Gragoatá, 24210-340 Niterói, RJ, Brasil 3PETROBRAS, CENPES, Av. Horácio Macedo, 950, Cidade Universitária, 21941-915 Rio de Janeiro, RJ, Brasil Manuscript received on September 24, 2013; accepted for publication on June 10, 2015 ABSTRACT This paper presents geochemical behaviour of trace elements of the felsic alkaline rocks of the state of Rio de Janeiro, Brazil, with special attention of fractional crystallization and continental crust assimilation. Fractionation of leucite and K-feldspar increases Rb/K and decreases K2O/(K2O+Na2O). Primitive nepheline syenite magmas have low Zr/TiO2, Sr, and Ba. On the Nb/Y vs. Zr/TiO2 diagram, these rocks are projected on the field of alkaline basalt, basanite, and nephelinite, instead of phonolite. Well-fractionated peralkaline nepheline syenite has high Zr/TiO2 but there are no zircon. The diagrams of silica saturation index (SSI) distinguish the trends originated form fractional crystallization and crustal assimilation. -

Chapter 10: Mantle Melting and the Generation of Basaltic Magma 2 Principal Types of Basalt in the Ocean Basins Tholeiitic Basalt and Alkaline Basalt
Chapter 10: Mantle Melting and the Generation of Basaltic Magma 2 principal types of basalt in the ocean basins Tholeiitic Basalt and Alkaline Basalt Table 10.1 Common petrographic differences between tholeiitic and alkaline basalts Tholeiitic Basalt Alkaline Basalt Usually fine-grained, intergranular Usually fairly coarse, intergranular to ophitic Groundmass No olivine Olivine common Clinopyroxene = augite (plus possibly pigeonite) Titaniferous augite (reddish) Orthopyroxene (hypersthene) common, may rim ol. Orthopyroxene absent No alkali feldspar Interstitial alkali feldspar or feldspathoid may occur Interstitial glass and/or quartz common Interstitial glass rare, and quartz absent Olivine rare, unzoned, and may be partially resorbed Olivine common and zoned Phenocrysts or show reaction rims of orthopyroxene Orthopyroxene uncommon Orthopyroxene absent Early plagioclase common Plagioclase less common, and later in sequence Clinopyroxene is pale brown augite Clinopyroxene is titaniferous augite, reddish rims after Hughes (1982) and McBirney (1993). Each is chemically distinct Evolve via FX as separate series along different paths Tholeiites are generated at mid-ocean ridges Also generated at oceanic islands, subduction zones Alkaline basalts generated at ocean islands Also at subduction zones Sources of mantle material Ophiolites Slabs of oceanic crust and upper mantle Thrust at subduction zones onto edge of continent Dredge samples from oceanic crust Nodules and xenoliths in some basalts Kimberlite xenoliths Diamond-bearing pipes blasted up from the mantle carrying numerous xenoliths from depth Lherzolite is probably fertile unaltered mantle Dunite and harzburgite are refractory residuum after basalt has been extracted by partial melting 15 Tholeiitic basalt 10 5 Figure 10-1 Brown and Mussett, A. E. (1993), The Inaccessible Earth: An Integrated View of Its Lherzolite Structure and Composition. -

Partial Melting - Simple Process, Huge Global Impact How Partial Melting, Coupled with Plate Tectonics, Has Changed the Chemistry of Our Planet
Earthlearningidea - http://www.earthlearningidea.com/ Partial melting - simple process, huge global impact How partial melting, coupled with plate tectonics, has changed the chemistry of our planet Demonstrating partial melting Explaining the planetary effects of partial melting Prepare two small beakers as described in the Each time that partial melting takes place during different resources section below. stages of the plate tectonic cycle, materials with different chemical and physical makeup are formed. Pre- prepared The starting point for these processes is the mantle, beakers where the most abundant elements are oxygen, silicon, magnesium and iron in that order. However the Earth’s crust contains much more silicon and oxygen and much less magnesium and iron than the mantle, and is formed through these stages: Photo: C. King. Stage 1: the mantle partially melts beneath oceanic ridges; the melt formed is richer in oxygen/silicon (and poorer in iron/magnesium) than the original mantle rock. This rises and then cools to form new oceanic crust and Show the pupils the beaker containing the mixture of ocean ridge volcanoes, as plates move apart. gravel and chopped candlewax before heating; ask what will happen if the beaker is heated until the wax Stage 2: the oceanic crust subducted beneath melts. Most will realise that the gravel will sink to the oceanic plates partially melts; the melt is still richer in bottom to form a mixed gravel/wax layer, whilst pure oxygen/silicon (and poorer in iron/ magnesium) than the wax will flow to the top to form another layer. Then original oceanic crust rock. -

Experimental Partitioning of Rb, Cs, Sr, and Ba Between Alkali Feldspar and Peraluminous Melt
American Mineralogist, Volume 81, pages 719-734,1996 Experimental partitioning of Rb, Cs, Sr, and Ba between alkali feldspar and peraluminous melt JONATHAN IcENHOWER AND DAVID LoNDON School of Geology and Geophysics, University of Oklahoma, 100 East Boyd Street, SEC 810, Norman, Oklahoma 73019, U.S.A. ABSTRACT Hydrous partial melting experiments performed between 650 and 750 °C at 200 MPa (H20) on synthetic metapelite compositions (quartz + albite + muscovite + biotite min- eral mixtures) doped with Ba, Sr, Rb, and Cs yielded alkali feldspar crystals with a wide range of compositions in equilibrium at their rims with peraluminous melt. Measured partition coefficients for normally trace lithophile elements between feldspar and melt [D(M)FSP/gl,M = Ba, Sr, Rb, Cs] do not depend on either temperature or bulk composition of melt for the compositions studied. Values of D(Sr)FSP/glare between 10 and 14 and appear to be independent of the albite and orthoclase contents of the feldspar crystals. In contrast, values of D(Ba)FSP/gland D(Rb)FsP/glare strongly dependent on the orthoclase content of feldspar, relationships that can be expressed by the following linear equations: D(Ba)FSP/gl= 0.07 + 0.25(orthoclase) and D(Rb)FsP/gl= 0.03 + 0.0 1(orthoclase), where orthoclase is in mole percent. These equations reproduce the range of previously reported values for D(Ba) and D(Rb) determined on natural and synthetic samples. A single par- tition coefficient for Cs was also determined at D(CS)Fsp/gl= 0.13. These data can be used in conjunction with recently published partition coefficients for muscovite, biotite, and plagioclase feldspars (Blundy and Wood 1991; Icenhower and London 1995) to model quantitatively the trace element signatures of peraluminous mag- mas during anatexis and crystallization. -

Petrogenesis of Slab-Derived Trondhjemite-Tonalite-Dacite/ Adakite Magmas M
Transactions of the Royal Society of Edinburgh: Earth Sciences, 87, 205-215, 1996 Petrogenesis of slab-derived trondhjemite-tonalite-dacite/ adakite magmas M. S. Drummond, M. J. Defant and P. K. Kepezhinskas ABSTRACT: The prospect of partial melting of the subducted oceanic crust to produce arc magmatism has been debated for over 30 years. Debate has centred on the physical conditions of slab melting and the lack of a definitive, unambiguous geochemical signature and petrogenetic process. Experimental partial melting data for basalt over a wide range of pressures (1-32 kbar) and temperatures (700-1150=C) have shown that melt compositions are primarily trondhjemite- tonalite-dacite (TTD). High-Al (> 15% A12O3 at the 70% SiO2 level) TTD melts are produced by high-pressure 015 kbar) partial melting of basalt, leaving a restite assemblage of garnet + clinopyroxe'ne ± hornblende. A specific Cenozoic high-Al TTD (adakite) contains lower Y, Yb and Sc and higher Sr, Sr/Y, La'/Yb and.Zr/Sm relative to other TTD types and is interpreted to represent a slab melt under garnet amphibolite to eclogite conditions. High-Al TTD with an adakite-like geochemical character is prevalent in the Archean as the result of a higher geotherm that facilitated slab melting. Cenozoic adakite localities are commonly associated with the subduction of young (<25Ma), hot oceanic crust, which may provide a slab geotherm (*9-10=C km"1) conducive for slab dehydration melting. Viable alternative or supporting tectonic effects that may enhance slab melting include highly oblique convergence and resultant high shear stresses and incipient subduction into a pristine hot mantle wedge. -
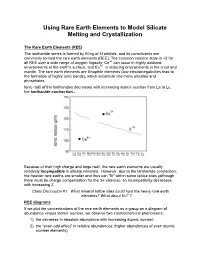
Using Rare Earth Elements to Model Silicate Melting and Crystallization
Using Rare Earth Elements to Model Silicate Melting and Crystallization The Rare Earth Elements (REE) The lanthanide series is formed by filling of 4f orbitals, and its constituents are commonly termed the rare earth elements (REE). The common valence state is +3 for all REE over a wide range of oxygen fugacity; Ce+4 can occur in highly oxidized environments at the earth's surface, and Eu+2 in reducing environments in the crust and mantle. The rare earth elements are lithophile elements (low electronegativities lead to the formation of highly ionic bonds), which substitute into many silicates and phosphates. Ionic radii of the lanthanides decreases with increasing atomic number from La to Lu, the lanthanide contraction… Because of their high charge and large radii, the rare earth elements are usually relatively incompatible in silicate minerals. However, due to the lanthanide contraction, the heavier rare earths are smaller and thus can "fit" within some lattice sites (although there must be charge compensation for the 3+ valence), so incompatibility decreases with increasing Z. Class Discussion #1: What mineral lattice sites could host the heavy rare earth elements? What about Eu2+? REE diagrams If we plot the concentrations of the rare earth elements as a group on a diagram of abundance versus atomic number, we observe two cosmochemical phenomena: 1) the decrease in absolute abundance with increasing atomic number; 2) the “even-odd effect” in relative abundances (higher abundances of even atomic number elements). Because these cosmochemical effects are common to all terrestrial rocks, we would rather ignore them in order to accentuate more subtle variations in absolute and relative concentrations of REE between samples. -
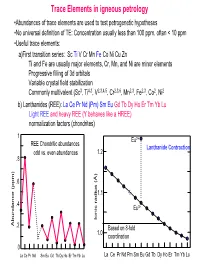
Trace Elements in Igneous Petrology
Trace Elements in igneous petrology •Abundances of trace elements are used to test petrogenetic hypotheses •No universal definition of TE: Concentration usually less than 100 ppm, often < 10 ppm •Useful trace elements: a)First transition series: Sc Ti V Cr Mn Fe Co Ni Cu Zn Ti and Fe are usually major elements, Cr, Mn, and Ni are minor elements Progressive filling of 3d orbitals Variable crystal field stabilization Commonly multivalent (Sc3, Ti4,3, V2,3,4,5, Cr2,3,6, Mn2,3, Fe2,3, Co2, Ni2 b) Lanthanides (REE): La Ce Pr Nd (Pm) Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Light REE and heavy REE (Y behaves like a HREE) normalization factors (chondrites) 1 Eu2+ REE Chondritic abundances Lanthanide Contraction odd vs. even abundances 1.2 .8 .6 1.1 .4 Eu3+ Ionic radius (Å) Ionic radius Abundance (ppm) .2 Based on 8-fold 1.0 coordination 0 La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu (c) Large Ion Lithophile Elements (LILE): may also be partitioned into fluid phase Alkalis: K Rb Cs (monovalent) Alkaline earths: Ba Sr (divalent) Actinides: U, Th, Ra, Pa (multiple valency) (d) High field strength elements (HFSE): small, highly-charged ions Zr, Hf (4 valent) Nb, Ta (4 and 5 valent) (e) Chalcophile elements: Cu, Zn, Pb, Ag, Hg, PGE, (Fe, Co, Ni) (f) Siderophile elements: Fe, Ni, Co, Ge, P, Ga, Au (PGE)… • Decoupled from major elements: lack of stoichiometric constraints (not strictly true) • Goldschmidt’s Rules • Generalities: Incompatible elements are elements that tend to be excluded from common minerals (olivines, pyroxenes, garnets, feldspars, oxides…) in equilibrium with a melt, i.e., they have low D values. -

Implications of Subduction Rehydration for Earth's Deep Water
Implications of Subduction Rehydration for Earth’s Deep Water Cycle Lars Rüpke Physics of Geological Processes, University of Oslo, Oslo, Norway, and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jason Phipps Morgan Cornell University, Ithaca, New York, USA and SFB 574 Volatiles and Fluids in Subduction Zones, Kiel, Germany Jacqueline Eaby Dixon RSMAS/MGG, University of Miami, Miami, Florida, USA The “standard model” for the genesis of the oceans is that they are exhalations from Earth’s deep interior continually rinsed through surface rocks by the global hydrologic cycle. No general consensus exists, however, on the water distribution within the deeper mantle of the Earth. Recently Dixon et al. [2002] estimated water concentrations for some of the major mantle components and concluded that the most primitive (FOZO) are significantly wetter than the recycling associated EM or HIMU mantle components and the even drier depleted mantle source that melts to form MORB. These findings are in striking agreement with the results of numerical modeling of the global water cycle that are presented here. We find that the Dixon et al. [2002] results are consistent with a global water cycle model in which the oceans have formed by efficient outgassing of the mantle. Present-day depleted mantle will contain a small volume fraction of more primitive wet mantle in addition to drier recycling related enriched components. This scenario is consis- tent with the observation that hotspots with a FOZO-component in their source will make wetter basalts than hotspots whose mantle sources contain a larger fraction of EM and HIMU components. -

The Transition-Zone Water Filter Model for Global Material Circulation: Where Do We Stand?
The Transition-Zone Water Filter Model for Global Material Circulation: Where Do We Stand? Shun-ichiro Karato, David Bercovici, Garrett Leahy, Guillaume Richard and Zhicheng Jing Yale University, Department of Geology and Geophysics, New Haven, CT 06520 Materials circulation in Earth’s mantle will be modified if partial melting occurs in the transition zone. Melting in the transition zone is plausible if a significant amount of incompatible components is present in Earth’s mantle. We review the experimental data on melting and melt density and conclude that melting is likely under a broad range of conditions, although conditions for dense melt are more limited. Current geochemical models of Earth suggest the presence of relatively dense incompatible components such as K2O and we conclude that a dense melt is likely formed when the fraction of water is small. Models have been developed to understand the structure of a melt layer and the circulation of melt and vola- tiles. The model suggests a relatively thin melt-rich layer that can be entrained by downwelling current to maintain “steady-state” structure. If deep mantle melting occurs with a small melt fraction, highly incompatible elements including hydro- gen, helium and argon are sequestered without much effect on more compatible elements. This provides a natural explanation for many paradoxes including (i) the apparent discrepancy between whole mantle convection suggested from geophysical observations and the presence of long-lived large reservoirs suggested by geochemical observations, (ii) the helium/heat flow paradox and (iii) the argon paradox. Geophysical observations are reviewed including electrical conductivity and anomalies in seismic wave velocities to test the model and some future direc- tions to refine the model are discussed. -

End of Chapter Question Answers Chapter 4 Review Questions 1
End of Chapter Question Answers Chapter 4 Review Questions 1. Describe the three processes that are responsible for the formation of magma. Answer: Magmas form from melting within the Earth. There are three types of melting: decompression melting, where magmas form when hot rock from deep in the mantle rises to shallower depths without undergoing cooling (the decrease in pressure facilitates the melting process); flux melting, where melting occurs due to the addition of volatiles such as CO2 and H2O; and heat transfer melting, where melting results from the transfer of heat from a hotter material to a cooler one. 2. Why are there so many different compositions of magma? Does partial melting produce magma with the same composition as the magma source from which it was derived? Answer: Magmas are formed from many different chemical constituents. Partial melting of rock yields magma that is more felsic (silicic) than the magma source because a higher proportion of chemicals needed to form felsic minerals diffuse into the melt at lower temperatures. Magma may incorporate chemicals dissolved from the solid rock through which it rises or from blocks of rock that fall into the magma. This process is called assimilation. Finally, fractional crystallization can modify magma composition as minerals crystallize out of a melt during the cooling process, causing the residual liquid to become progressively more felsic. 3. Why does magma rise from depth to the surface of the Earth? Answer: Magma rises toward the surface of the Earth because it is less dense than solid rock and buoyant relative to its surroundings. -

Incompatible Element Ratios in Plate Tectonic and Stagnant Lid Planets Kent C. Condie1 and Charles K. Shearer 2. 1Department Of
47th Lunar! and Planetary Science Conference (2016) 1058.pdf Incompatible Element Ratios in Plate Tectonic and Stagnant Lid Planets Kent C. Condie1 and Charles K. Shearer2. 1Department of Earth and Environmental Science, New Mexico Tech, Socorro, New Mexico 87801, 2 Institute of Meteoritics, Department of Earth and Planetary Science, University of New Mexico, Albuquerque, New Mexico 87131, 2 NASA Johnson Space Center, Houston, TX 77058. Introduction: Because ratios of elements with group centered near Earth’s primitive mantle (PM) similar incompatibilities do not change much with composition (Fig. 2). This distribution is similar to degree of melting or fractional crystallization in the the distribution of most mare basalts from the Moon mantles of silicate planets, they are useful in sampled by the Apollo missions, except for the high- characterizing compositions of mantle sources of Ti basalts from Apollo 11 and 17 both of which are basalts that have not interacted with continental crust enriched in Nb. The primitive mantle grouping is [1,2]. Using Nb/Th and Zr/Nb ratios in young especially evident for Apollo 11, 12 and 15 basalts in oceanic basalts, three mantle domains can be which samples cluster tightly around primitive identified [3]: enriched (EM), depleted (DM), and mantle (PM) composition on Zr/Nb-Nb/Th, Th/Yb- hydrated mantle (HM) (Fig. 1). DM is characterized Nb/Yb and La/Sm-Nb/Th diagrams (Fig. 3). Very by high values of both ratios (Nb/Th > 8; Zr/Nb > 20) low-Ti green volcanic glasses (and some due to Th depletion relative to Nb, and to Nb intermediate-Ti yellow volcanic glasses) plot in a depletion relative to Zr. -

Durham Research Online
Durham Research Online Deposited in DRO: 14 March 2018 Version of attached le: Accepted Version Peer-review status of attached le: Peer-reviewed Citation for published item: Namur, Olivier and Humphreys, Madeleine C.S. (2018) 'Trace element constraints on the dierentiation and crystal mush solidication in the Skaergaard intrusion, Greenland.', Journal of petrology., 59 (3). pp. 387-418. Further information on publisher's website: https://doi.org/10.1093/petrology/egy032 Publisher's copyright statement: This is a pre-copyedited, author-produced version of an article accepted for publication in Journal Of Petrology following peer review. The version of record Namur, Olivier Humphreys, Madeleine C.S. (2018). Trace element constraints on the dierentiation and crystal mush solidication in the Skaergaard intrusion, Greenland. Journal of Petrology is available online at: https://doi.org/10.1093/petrology/egy032. Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full DRO policy for further details. Durham University Library, Stockton Road, Durham DH1 3LY, United Kingdom Tel : +44 (0)191 334 3042 | Fax : +44 (0)191 334 2971 https://dro.dur.ac.uk Trace element constraints on the differentiation and crystal mush solidification in the Skaergaard intrusion, Greenland Olivier Namur1*, Madeleine C.S.