Historic and Modern Utilities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Behavior of Mixtures of Heavy Oil Stocks and Waxes
BEHAVIOR OF MIXTURES OF HEAVY OIL STOCKS AND WAXES A Thesis Submitted to the College of Engineering of Nahrain University in Partial Fulfillment of the Requirements for the Degree of Master of Science in Chemical Engineering by SARMAD KAMAL FAKHRALDEEN (B. Sc. in Chemical Engineering 2004) Thul - Hijjah 1428 December 2007 Abstract Experimental work is carried out to study behavior of mixture of mixing of two types of waxes (paraffin and microcrystalline) and different types of heavy oil (base stock 40, base stock 60, base stock 150, furfural extract 60 and furfural extract 150). The experimental work included the determination of important properties of mixture which are drop point, penetration, and copper corrosion. Also the experimental work included the investigation of the effect of wax weight percentage, wax type and heavy oil type on these properties of mixture. Various types of mixtures were used containing different percentages of waxes and heavy oil. The results revealed that the wax weight percentage and oil type have considerable effect on the drop point, penetration and copper corrosion of mixtures. It is found that increasing wax weight percentage leads to increase in the drop point for all types of mixture. The penetration and copper corrosion are found to decrease with the increase in wax weight percentage. Generally the microcrystalline wax exhibits higher drop point than the paraffin wax. Also the microcrystalline wax gives lower penetration and copper corrosion than paraffin wax in all heavy oil types. The value of drop point of different types of mixtures increases in the following manner for same type of wax and wax weight percentage: base stock oil 40, base stock oil 60, furfural extract oil 60, base stock oil 150 and I furfural extract oil 150, The value of penetration of mixtures and increases in the following manner for same type of wax and wax weight percentage: furfural extract oil 150, base stock oil 150, furfural extract oil 60, base stock oil 60 and base stock oil 40. -

Infrared Spectra of Noble Gases (12000 to 19000 Ar Curtis 1
Journal of Research of the Nationa l Bureau of Sta ndards Vol. 49, No. 2, August 1952 Resea rch Paper 2345 Infrared Spectra of Noble Gases (12000 to 19000 Ar Curtis 1. Humphreys and Henry 1. Kostkowski . The first spectra of heliUl.n, neon, argon, krypton, and xe non, excited by discharges in gelssler t ubes, operated by direct connection to a transformer, have been explored in the ll1frared (1 2090 to 19000 A) . A hi~h-reso lu t.i o n , automatically recording, infrared spec trom eter, emploYlllg a. 15009-h~ es -p e r-lllch gratlllg and lead-sulfi de photocond ucting detector, was used as t he dlspersmg mstrument. A new set of wavelength values is reported for all t hese spectra. New data include 18 pre viously unreported lines of neon and 36 of krypton all of which have been. classified . .~h e descriptions of t he spectra of argon, krypton, and xe non represent essent ially a repetit IOn of t he observations of Sitt ner and Peck. Several prev io~ s l y missing classificat ions a re supplied, also a few amended interpretat ions. The analysIs of t hese spectra m ay be regarded as complete. Use of selected lines as wavelength standards is suggested., 1. Introduction mocouple detector were reported by Humphreys and Plyler [2] . These observations covered the same The essentially complete character of both the spec tral region in which the data herein reported were d escription and interpretation of th e photographed obtained, but, because of well-known limitations af spec tra of the noble atmospheric gases makes it fecting the precision of spectral data obtained by apparent that any reopening of th e subject can be prism spec trometers with thermal detectors, may be justified only on the basis of th e availability of new' considered as en tirely sup erseded by the presen t sources of information, such as a new technique of work. -

Nikola Tesla
Nikola Tesla Nikola Tesla Tesla c. 1896 10 July 1856 Born Smiljan, Austrian Empire (modern-day Croatia) 7 January 1943 (aged 86) Died New York City, United States Nikola Tesla Museum, Belgrade, Resting place Serbia Austrian (1856–1891) Citizenship American (1891–1943) Graz University of Technology Education (dropped out) ‹ The template below (Infobox engineering career) is being considered for merging. See templates for discussion to help reach a consensus. › Engineering career Electrical engineering, Discipline Mechanical engineering Alternating current Projects high-voltage, high-frequency power experiments [show] Significant design o [show] Awards o Signature Nikola Tesla (/ˈtɛslə/;[2] Serbo-Croatian: [nǐkola têsla]; Cyrillic: Никола Тесла;[a] 10 July 1856 – 7 January 1943) was a Serbian-American[4][5][6] inventor, electrical engineer, mechanical engineer, and futurist who is best known for his contributions to the design of the modern alternating current (AC) electricity supply system.[7] Born and raised in the Austrian Empire, Tesla studied engineering and physics in the 1870s without receiving a degree, and gained practical experience in the early 1880s working in telephony and at Continental Edison in the new electric power industry. He emigrated in 1884 to the United States, where he became a naturalized citizen. He worked for a short time at the Edison Machine Works in New York City before he struck out on his own. With the help of partners to finance and market his ideas, Tesla set up laboratories and companies in New York to develop a range of electrical and mechanical devices. His alternating current (AC) induction motor and related polyphase AC patents, licensed by Westinghouse Electric in 1888, earned him a considerable amount of money and became the cornerstone of the polyphase system which that company eventually marketed. -

Combustion and Heat Release Characteristics of Biogas Under Hydrogen- and Oxygen-Enriched Condition
energies Article Combustion and Heat Release Characteristics of Biogas under Hydrogen- and Oxygen-Enriched Condition Jun Li 1, Hongyu Huang 2,*, Huhetaoli 2, Yugo Osaka 3, Yu Bai 2, Noriyuki Kobayashi 1,* and Yong Chen 2 1 Department of Chemical Engineering, Nagoya University, Nagoya, Aichi 464-8603, Japan; [email protected] 2 Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, China; [email protected] (H.); [email protected] (Y.B.); [email protected] (Y.C.) 3 Faculty of Mechanical Engineering, Kanazawa University, Kakuma, Kanazawa, Ishikawa 920-1192, Japan; [email protected] * Correspondence: [email protected] (H.H.); [email protected] (N.K.); Tel.: +86-20-870-48394 (H.H.); +81-52-789-5428 (N.K.) Received: 10 May 2017; Accepted: 20 July 2017; Published: 13 August 2017 Abstract: Combustion and heat release characteristics of biogas non-premixed flames under various hydrogen-enriched and oxygen-enriched conditions were investigated through chemical kinetics simulation using detailed chemical mechanisms. The heat release rates, chemical reaction rates, and molar fraction of all species of biogas at various methane contents (35.3–58.7%, mass fraction), hydrogen addition ratios (10–50%), and oxygen enrichment levels (21–35%) were calculated considering the GRI 3.0 mechanism and P1 radiation model. Results showed that the net reaction rate of biogas increases with increasing hydrogen addition ratio and oxygen levels, leading to a higher net heat release rate of biogas flame. Meanwhile, flame length was shortened with the increase in hydrogen addition ratio and oxygen levels. -

Intro to Light Fixtures
Intro to Light Fixtures IN THE BEGINNING PRIMITIVE LAMPS - (c 13,000 BC to 3,000 BC) Prehistoric man, used primitive lamps to illuminate his cave. These lamps, made from naturally occurring materials, such as rocks, shells, horns and stones, were filled with grease and had a fiber wick. Lamps typically used animal or vegetable fats as fuel. In the ancient civilizations of Babylonian and Egypt, light was a luxury. The Arabian Nights were far from the brilliance of today. The palaces of the wealthy were lighted only by flickering flames of simple oil lamps. These were usually in the form of small open bowls with a lip or spout to hold the wick. Animal fats, fish oils or vegetable oils (palm and olive) furnished the fuels. Early Developments Early Developments Rush lights: Candles: Tall, grass-like plant dipped in fat Most expensive candles made of beeswax Most common in churches and homes of nobility Snuffers cut the wick while maintaining the flame Early Developments Early Developments New Developments There was a need to improve the light several ways: 1. The need for a constant flame, which could me left unattended for a longer period of time 2. Decrease heat and smoke for interior use 3. To increase the light output 4. An easier way to replenish the source….thus, the development of gas and electricity 5. Produce light with little waste or conserve energy Page 1 Intro to Light Fixtures Industrial Revolution - Europe Gas lamps developed: London well known for gas lamps Argand Lamp Eiffel Tower (1889) originally used gas lamps The Argand burner, which was introduced in 1784 by the Swiss inventor Argand, was a major improvement in brightness compared to traditional open-flame oil lamps. -

Confidential Ssociates a History of Energy Part I
Winter 2014 Luthin Confidential ssociates A History of Energy Part I History of Lighting - Part 1 It wasn’t until the late In the early 1800s, gas M el Brooks’ 2000- 1700’s that European light (initially at less than Inside this issue: year old man used oil lamps (at ~0.3 lu- 1 lumen/watt), used coal torches in his cave, but mens/watt) became gas or natural gas from History of Lighting Part 1 1 today’s lighting is a tad widely available and mines or wells, and was more sophisticated, accepted due to im- relatively common in ur- How to Become A Producer 2 having gone through provements in design ban England. Its use ex- half a dozen stages, and the whaling indus- panded rapidly after the Can Spaceballs Be Shot Out 3 each producing more try’s ability to produce development of the incan- of Earth Tubes? light out of less energy sperm oil. That refined descent gas mantle around i.e., efficacy, than its product burned cleanly, 1890. That device more High (Voltage) Anxiety 3 predecessor. didn’t smell too bad, than doubled the efficacy and was relatively (to 2 lumens/watt) of gas On A Personal Note 4 Torches made of moss cheap compared to lighting, using a filament and animal fat, and commercially-made containing thorium and and the power industry adopted crude oil lamps were candles. cerium, which converted it as a standard. During this the mainstay for indoor more of the gas flame’s period, Nikola Tesla, and oth- lighting until the pro- The Industrial Revolu- heat into white light. -

Energy and the Hydrogen Economy
Energy and the Hydrogen Economy Ulf Bossel Fuel Cell Consultant Morgenacherstrasse 2F CH-5452 Oberrohrdorf / Switzerland +41-56-496-7292 and Baldur Eliasson ABB Switzerland Ltd. Corporate Research CH-5405 Baden-Dättwil / Switzerland Abstract Between production and use any commercial product is subject to the following processes: packaging, transportation, storage and transfer. The same is true for hydrogen in a “Hydrogen Economy”. Hydrogen has to be packaged by compression or liquefaction, it has to be transported by surface vehicles or pipelines, it has to be stored and transferred. Generated by electrolysis or chemistry, the fuel gas has to go through theses market procedures before it can be used by the customer, even if it is produced locally at filling stations. As there are no environmental or energetic advantages in producing hydrogen from natural gas or other hydrocarbons, we do not consider this option, although hydrogen can be chemically synthesized at relative low cost. In the past, hydrogen production and hydrogen use have been addressed by many, assuming that hydrogen gas is just another gaseous energy carrier and that it can be handled much like natural gas in today’s energy economy. With this study we present an analysis of the energy required to operate a pure hydrogen economy. High-grade electricity from renewable or nuclear sources is needed not only to generate hydrogen, but also for all other essential steps of a hydrogen economy. But because of the molecular structure of hydrogen, a hydrogen infrastructure is much more energy-intensive than a natural gas economy. In this study, the energy consumed by each stage is related to the energy content (higher heating value HHV) of the delivered hydrogen itself. -

Tracing the Recorded History of Thin-Film Sputter Deposition: from the 1800S to 2017
Review Article: Tracing the recorded history of thin-film sputter deposition: From the 1800s to 2017 Cite as: J. Vac. Sci. Technol. A 35, 05C204 (2017); https://doi.org/10.1116/1.4998940 Submitted: 24 March 2017 . Accepted: 10 May 2017 . Published Online: 08 September 2017 J. E. Greene COLLECTIONS This paper was selected as Featured ARTICLES YOU MAY BE INTERESTED IN Review Article: Plasma–surface interactions at the atomic scale for patterning metals Journal of Vacuum Science & Technology A 35, 05C203 (2017); https:// doi.org/10.1116/1.4993602 Microstructural evolution during film growth Journal of Vacuum Science & Technology A 21, S117 (2003); https://doi.org/10.1116/1.1601610 Overview of atomic layer etching in the semiconductor industry Journal of Vacuum Science & Technology A 33, 020802 (2015); https:// doi.org/10.1116/1.4913379 J. Vac. Sci. Technol. A 35, 05C204 (2017); https://doi.org/10.1116/1.4998940 35, 05C204 © 2017 Author(s). REVIEW ARTICLE Review Article: Tracing the recorded history of thin-film sputter deposition: From the 1800s to 2017 J. E. Greenea) D. B. Willett Professor of Materials Science and Physics, University of Illinois, Urbana, Illinois, 61801; Tage Erlander Professor of Physics, Linkoping€ University, Linkoping,€ Sweden, 58183, Sweden; and University Professor of Materials Science, National Taiwan University Science and Technology, Taipei City, 106, Taiwan (Received 24 March 2017; accepted 10 May 2017; published 8 September 2017) Thin films, ubiquitous in today’s world, have a documented history of more than 5000 years. However, thin-film growth by sputter deposition, which required the development of vacuum pumps and electrical power in the 1600s and the 1700s, is a much more recent phenomenon. -

!History of Lightingv2.Qxd
CONTENTS Introduction 3 The role of lighting in modern society 3 1. The oldest light sources 4 Before the advent of the lamp 4 The oldest lamps 4 Candles and torches 5 Further development of the oil lamp 6 2. Gaslight 9 Introduction 9 Early history 9 Gas production 10 Gaslight burners 10 The gas mantle 11 3. Electric lighting before the incandescent lamp 14 Introduction 14 Principle of the arc lamp 15 Further development of the arc lamp 16 Applications of the arc lamp 17 4. The incandescent lamp 20 The forerunners 20 The birth of the carbon-filament lamp 22 Further development of the carbon-filament lamp 25 Early metal-filament lamps 27 The Nernst lamp 28 The birth of the tungsten-filament lamp 29 Drawn tungsten filaments 30 Coiled filaments 30 The halogen incandescent lamp 31 5. Discharge lamps 32 Introduction 32 The beginning 32 High-voltage lamps 33 Early low-pressure mercury lamps 34 The fluorescent lamp 35 High-pressure mercury lamps 36 Sodium lamps 37 The xenon lamp 38 6. Electricity production and distribution 39 Introduction 39 Influence machines and batteries 39 Magneto-electric generators 40 Self-exciting generators 41 The oldest public electricity supply systems 41 The battle of systems 42 The advent of modern a.c. networks 43 The History of Light and Lighting While the lighting industry is generally recognized as being born in 1879 with the introduction of Thomas Alva Edison’s incandescent light bulb, the real story of light begins thousands of years earlier. This brochure was developed to provide an extensive look at one of the most important inventions in mankind’s history: artificial lighting. -

Optical Measurements of Atmospheric Aerosols: Aeolian Dust, Secondary Organic Aerosols, and Laser-Induced Incandescence of Soot
Optical Measurements of Atmospheric Aerosols: Aeolian Dust, Secondary Organic Aerosols, and Laser-Induced Incandescence of Soot by Lulu Ma, B. S. A Dissertation In Chemistry Submitted to the Graduate Faculty of Texas Tech University in Partial Fulfillment of the Requirements for the Degree of Doctor of philosophy Approved Jonathan E. Thompson Chair of Committee Dimitri Pappas Carol L. Korzeniewski Dominick Casadonte Interim Dean of the Graduate School August, 2013 Copyright 2013, Lulu Ma Texas Tech University, Lulu Ma, August 2013 ACKNOWLEDGMENTS I would like to thank my advisor, Dr. Thompson for his guidance and encouragement throughout the past 4 years in Texas Tech University. His profound knowledge, diligence and patience encouraged me and has setup a great example for me in the future. I also thank to Dr. Pappas and Dr. Korzeniewski for their help and for taking time as my committee members. I also would like to thank Dr. Ted Zobeck for his help on soil dust measurements and soil sample collection and also Dr. P. Buseck and his lab for TEM analysis. My group members: Hao Tang, Fang Qian, Kathy Dial, Haley Redmond, Yiyi Wei, Tingting Cao, and Qing Zhang, also helped me a lot in both daily life, and research. At last, I would like to thank my parents and friends for their love and continuous encouragement and support. ii Texas Tech University, Lulu Ma, August 2013 TABLE OF CONTENTS ACKNOWLEDGMENTS……………………………………………………………….ii ABSTRACT ……………………………………………………………………………..vi LIST OF TABLES …………………………………………………………………….viii LIST OF FIGURES……………………………………………………..………………ix LIST OF ABBREVIATIONS ………………………………………….………………xi I. INTRODUCTION ......................................................................................................... 1 1.1 Introduction to Atmospheric Aerosols. ................................................................... 1 1.1.1 Natural Sources ............................................................................................. -

Commentary Kerosene-Based Lighting: an Overlooked Source of Exposure to Household Air Pollution?
Feature: Air quality challenges in low-income settlements Commentary Kerosene-based lighting: an overlooked source of exposure to household air pollution? Ariadna Curto 1,2,3, Cathryn Tonne 1,2,3 1Barcelona Institute for Global Health (ISGlobal), Barcelona, Spain 2Universitat Pompeu Fabra (UPF), Barcelona, Spain 3CIBER Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain https://doi.org/10.17159/caj/2020/30/1.8420 Access to affordable, reliable, modern and sustainable energy is prolonged periods, resulting in high levels of inhaled pollutants one of the seventeen Sustainable Development Goals (SDG) set (i.e. more mass inhaled per mass emitted). An experimental by the United Nations for 2030. However, estimates indicate that study in Kenya estimated that night kiosk vendors can inhale progress towards that goal is not on track: 650 million people 1560 µg of fine particles per day emitted by kerosene lamps worldwide are estimated to remain without access to electricity alone (Apple et al., 2010). Relatively few studies have attempted in 2030 (IEA, IRENA, UNSD, WB, WHO, 2019). Nine out of ten of to quantify the contribution of kerosene-based lighting to these people will live in Sub-Saharan Africa (SSA), mostly in rural particle exposure in populations in SSA. We previously reported communities, which face barriers in terms of affordability and that kerosene-based lighting was the strongest determinant supply. of 24-h average and peak personal exposure to black carbon among women living in a semi-rural area of Mozambique (Curto Without access to affordable and reliable clean energy to et al., 2019). Women who used kerosene as the primary source meet daily cooking, lighting, and heating needs, individuals of lighting had 81% and 93% higher average and peak personal rely on inefficient fuels and technologies that give rise to black carbon exposure, respectively, than those using electricity. -
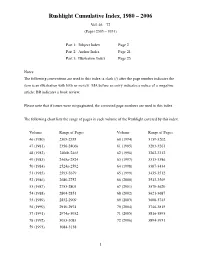
Rushlight Index 1980-2006
Rushlight Cumulative Index, 1980 – 2006 Vol. 46 – 72 (Pages 2305 – 3951) Part 1: Subject Index Page 2 Part 2: Author Index Page 21 Part 3: Illustration Index Page 25 Notes: The following conventions are used in this index: a slash (/) after the page number indicates the item is an illustration with little or no text. MA before an entry indicates a notice of a magazine article; BR indicates a book review. Please note that if issues were mispaginated, the corrected page numbers are used in this index. The following chart lists the range of pages in each volume of the Rushlight covered by this index. Volume Range of Pages Volume Range of Pages 46 (1980) 2305-2355 60 (1994) 3139-3202 47 (1981) 2356-2406a 61 (1995) 3203-3261 48 (1982) 2406b-2465 62 (1996) 3262-3312 49 (1983) 2465a-2524 63 (1997) 3313-3386 50 (1984) 2524a-2592 64 (1998) 3387-3434 51 (1985) 2593-2679 65 (1999) 3435-3512 52 (1986) 2680-2752 66 (2000) 3513-3569 53 (1987) 2753-2803 67 (2001) 3570-3620 54 (1988) 2804-2851 68 (2002) 3621-3687 55 (1989) 2852-2909 69 (2003) 3688-3745 56 (1990) 2910-2974 70 (2004) 3746-3815 57 (1991) 2974a-3032 71 (2005) 3816-3893 58 (1992) 3033-3083 72 (2006) 3894-3951 59 (1993) 3084-3138 1 Rushlight Subject Index Subject Page Andrews' burning fluid vapor lamps 3400-05 Abraham Gesner: Father of Kerosene 2543-47 Andrews patent vapor burner 3359/ Accessories for decorating lamps 2924 Andrews safety lamp, award refused 3774 Acetylene bicycle lamps, sandwich style 3071-79 Andrews, Solomon, 1831 gas generator 3401 Acetylene bicycle lamps, Solar 2993-3004