Long-Term Ecosystem Changes in Riparian Forests Ecological Research Monographs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Hornbeam Maple Acer Carpinifolium Rating: 0.0 ( 0 Votes)
Hornbeam maple Acer carpinifolium Rating: 0.0 ( 0 votes) This description is for Hornbeam maple (Acer carpinifolium): So named because the wood is tough like a beam made of horns; handy, should you ever need a beam made of horns but you're woefully low on horns. Acer carpinifolium, commonly known as the Hornbeam maple hails from Japan. A fairly small tree, growing no taller than about 10-15m. Unlike most other acers, this tree does not have lobed leaves. Instead it has ridged oval leaves with a serrated edge. They look similar to the leaves of the hornbean, hence the name. In the autumn this tree really shines as the leaves turn a vibrant shade of yellow. It's a very hardy tree that will grow in most soil types as long as it is fertile and well- drained. Find Hornbeam maple in our Shop! Free shipping from € 50! Plant Environment Usage Known dangers? Acidity Standard category no Acidic Trees & shrubs Neutral Shrubs Alkaline Height [m] Hardiness zone Grown for 5 - 6 Z4-7 Ornamental Foliage Plant Environment Usage Spread [m] Heat zone Creative category 4 H7-1 Kid Approved For Beginners Show-offs Dominant flower colour Winter temperatures [°C] Garden type Green -34 - -12 Woodland Park City Flower Fragrance Heat days Garden spaces No, neutral please 0 - 90 Specimen Flowering seasons Moisture Gardening expertise Early spring well-drained but frequently watered beginner Mid spring Foliage in spring Soil type Time to reach full size Green sandy up to 20 years Clay chalky loams Foliage in summer Sun requirements Green Full sun Partial shade Foliage in Autumn Exposure Red shades Sheltered Propagation methods grafting seed budding . -

Plants Unlimited Autumn Moon Full Moon Maple
[email protected] 207.594.7754 P.O. Box 374 629 Commercial St. Rockport, Maine 04856 Autumn Moon Full Moon Maple Acer shirasawanum 'Autumn Moon' Height: 20 feet Spread: 20 feet Sunlight: Hardiness Zone: 4b Description: This new introduction is taking the plant world by storm with its golden foliage tipped in rich red which lasts into summer, followed by vibrant fall color, the effect is stunning; an ideal accent for the bright home landscape Ornamental Features Autumn Moon Full Moon Maple foliage Autumn Moon Full Moon Maple has attractive Photo courtesy of NetPS Plant Finder tomato-orange-tipped chartreuse foliage which emerges scarlet in spring. The lobed leaves are highly ornamental and turn outstanding shades of gold and in the fall. Neither the flowers nor the fruit are ornamentally significant. Landscape Attributes Autumn Moon Full Moon Maple is a deciduous tree with a more or less rounded form. Its average texture blends into the landscape, but can be balanced by one or two finer or coarser trees or shrubs for an effective composition. This is a relatively low maintenance tree, and should only be pruned in summer after the leaves have fully Autumn Moon Full Moon Maple developed, as it may 'bleed' sap if pruned in late winter or Photo courtesy of NetPS Plant Finder early spring. It has no significant negative characteristics. Autumn Moon Full Moon Maple is recommended for the following landscape applications; - Accent - Shade - Mass Planting - Hedges/Screening Visit plants-unlimited.com [email protected] 207.594.7754 P.O. Box 374 629 Commercial St. -

Experience 2,000 Follow the Path from the Roundabout Towards East, Past the Small Lake and You Will Be at the Main Entrance
The Arboretum in Hørsholm Why an Arboretum? Te Arboretum is a living collection of more than 8,500 trees Less than a hundred species of trees, shrubs and woody climbers and shrubs. Te objective of the collection is to include and are native to Denmark. Te main reason for this is the fact that observe woody plant species that can survive and grow under most of Northern Europe during each of the last 7 major glacia- Danish conditions. tions was covered by ice, and the unglaciated landscapes north of the Alps were covered by arctic and sub-arctic tundras and steppes. All plants in the Arboretum have detailed documentation of age, Te rich fora in e.g. North America and Eastern Asia could escape GPS location, species identity, and the geographic origin of where such cold and dry climates. seeds were collected. Te information is stored in a database and can be accessed from the Arboretum webpage. In spite of our poor native dendrofora, a rich variety of exotic species and provenances are able to grow in the Danish climate. Te collection is managed by the Department of Geosciences At present the collections of the Arboretum include approximately Cornus kousa and Natural Resource Management, University of Copenhagen. 2,000 species, subspecies and cultivars in an area of 25 ha. Te Arboretum is a continuation of the Forest Botanical Garden in reveals if the seed was collected from a natural population (W), Charlottenlund from 1838. Te establishment of the Arboretum in from a plant that originates from a natural population (Z) Hrsholm was initiated in 1936, to support teaching and research or is a cultivated variety (G). -

Specializing in Rare and Unique Trees 2020 Catalogue
Whistling Gardens Ltd., 698 Concession 3, Wilsonville, ON N0E 1Z0 Phone: 519-443-5773 Fax: 519-443-4141 Email: [email protected] Specializing in Rare and Unique Trees 2020 Catalogue Pot sizes: The number represents the size of the pot ie. #1= 1 gallon, #10 = 10 gallon #1 potted conifers are usually 3-5years old. #10 potted conifers dwarf conifers are between 10 and 15 years old #1 trees= usually seedlings #10 trees= can be several years old anywhere from 5 to 10' tall depending on species and variety. Please ask us on sizes and varieties you are not sure about. Many plants are limited to 1 specimen. To reserve your plant(s) a 25% is required. Plants should be picked up by June 15th. Most plants arrive at the gardens by May 10th. Guarantee: We cannot control the weather (good or bad), rodents (big or small), pests (teenie, tiny), poor siting, soil types, lawnmovers, snowplows etc. Plants we carry are expected to grow within the parameters of normal weather conditons. All woody plant purchases are guaranteed from time of purchase to December 1st of current year. Perennials are not guaranteed. Any plant not performing or dying in current season will be happily replaced or credited towards a new plant. Please email us if possible with any info needed about our plants. We do not have a phone in the garden centre and I'm rarely in the office. It is very helpful to copy and paste the botanical name of the plant into your Google browser, in most cases, a detailed summary with photos is given. -

Arboretum News Armstrong News & Featured Publications
Georgia Southern University Digital Commons@Georgia Southern Arboretum News Armstrong News & Featured Publications Spring 2019 Arboretum News Georgia Southern University- Armstrong Campus Follow this and additional works at: https://digitalcommons.georgiasouthern.edu/armstrong-arbor-news Part of the Education Commons This article is brought to you for free and open access by the Armstrong News & Featured Publications at Digital Commons@Georgia Southern. It has been accepted for inclusion in Arboretum News by an authorized administrator of Digital Commons@Georgia Southern. For more information, please contact [email protected]. Arboretum News Issue 9 | Spring 2019 A Newsletter of the Georgia Southern University Armstrong Campus Arboretum From the Editor: Arboretum News, published by the Grounds Operations Department ’d like to introduce you to the Armstrong Arboretum of the new of Georgia Southern University- IGeorgia Southern University-Armstrong Campus. Designated Armstrong Campus, is distributed as an on-campus arboretum in 2001 by former Armstrong to faculty, staff, students and Atlantic State University president Dr. Thomas Jones, the friends of the Armstrong Arboretum. The Arboretum university recognized the rich diversity of plant life on campus. encompasses Armstrong’s 268- The Arboretum continues to add to that diversity and strives to acre campus and displays a wide function as a repository for the preservation and the conservation variety of shrubs and other woody of plants from all over the world. We also hope to inspire students, plants. Developed areas of campus faculty, staff and visitors to appreciate the incredible diversity contain native and introduced species of trees and shrubs. Most that plants have to offer. -
Environmental Factors Controlling the Distribution of Forest Plants with Special Reference to Floral Mixture in the Boreo-Nemora
Environmental Factors Controlling the Distribution of Forest Plants with Special Reference to Floral Mixture in the Title Boreo-Nemoral Ecotone, Hokkaido Island Author(s) Uemura, Shigeru Environmental science, Hokkaido University : journal of the Graduate School of Environmental Science, Hokkaido Citation University, Sapporo, 15(2), 1-54 Issue Date 1993-03-25 Doc URL http://hdl.handle.net/2115/37276 Type bulletin (article) File Information 15(2)_1-54.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP 1 Environ,Sci.,I'Iokl{aidoUniversity 15(2) 1-54 Dec,1992 Environmental Factors Controlling the Distribution of Forest PlaRts with Special Reference to Floral Mixture in the Boreo-Nemoral EcotoRe, Hokkaido Island Shigeru Uemura Department of Biosystem Management, Division of Environmental Conservation, Graduate School of Environmental Science, Hokkaiclo University, Sapporo 060, Japan Abstract Effects of climatic factors on the plant distribution were examined by means of direct gradient analysis, and the relationship of forest flora with Iife form and phytogeographical distribution was exaniined. Subsequently, leaf phenology of forest plants were analyzed to evaluate the adaptive signifi- cance in relation to the environments in forest understory. In the boreo-nemoral forest ecotone, Kokkaido Island, northern Japan, co-occurrence of northern and southern plants in a certain forest site is more notable in the understory than in the crown, and this dates back to the late--Quaternary period, where the decrease in temperature associated with the glacial period forced the unclerstory plants to adapt their life forms or leaf habits to snowcover and to light conditions of the interior forests, I<ey words: Direct gradient analysis; Floral mixture; Leaf phenology; Mixed forest; Phytogoegraphy; Snowcover; Understory Intoroduetion In the upper-middle latitudes of Europe, eastern Asia and eastern North America, the boreal coniferous forest formation confronts to the temperate hardwood forest formation. -

Campus Tree Care Plan
2015 Illinois Tree Campus A Tree Care Plan for the University of Illinois at Urbana-Champaign December 30, 2015 Contents Contents .................................................................................................................................................................................... 3 Standard 1: Campus Tree Advisory Committee .................................................................................................................. 1 Background .......................................................................................................................................................................... 1 Campus Tree Advisory Committee .................................................................................................................................. 1 2015 Committee Members ................................................................................................................................................. 2 2015 Meeting Schedule ....................................................................................................................................................... 2 Standard 2: Campus Tree Care Plan...................................................................................................................................... 2 1. Purpose of Tree Care Plan ............................................................................................................................................. 2 2. Responsible Department ............................................................................................................................................... -

Flood Loss Model Model
GIROJ FloodGIROJ Loss Flood Loss Model Model General Insurance Rating Organization of Japan 2 Overview of Our Flood Loss Model GIROJ flood loss model includes three sub-models. Floods Modelling Estimate the loss using a flood simulation for calculating Riverine flooding*1 flooded areas and flood levels Less frequent (River Flood Engineering Model) and large- scale disasters Estimate the loss using a storm surge flood simulation for Storm surge*2 calculating flooded areas and flood levels (Storm Surge Flood Engineering Model) Estimate the loss using a statistical method for estimating the Ordinarily Other precipitation probability distribution of the number of affected buildings and occurring disasters related events loss ratio (Statistical Flood Model) *1 Floods that occur when water overflows a river bank or a river bank is breached. *2 Floods that occur when water overflows a bank or a bank is breached due to an approaching typhoon or large low-pressure system and a resulting rise in sea level in coastal region. 3 Overview of River Flood Engineering Model 1. Estimate Flooded Areas and Flood Levels Set rainfall data Flood simulation Calculate flooded areas and flood levels 2. Estimate Losses Calculate the loss ratio for each district per town Estimate losses 4 River Flood Engineering Model: Estimate targets Estimate targets are 109 Class A rivers. 【Hokkaido region】 Teshio River, Shokotsu River, Yubetsu River, Tokoro River, 【Hokuriku region】 Abashiri River, Rumoi River, Arakawa River, Agano River, Ishikari River, Shiribetsu River, Shinano -
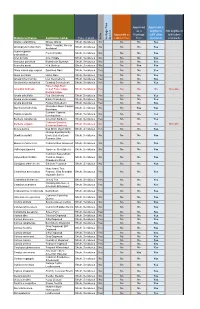
Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Acer Carpinifolium (Hornbeam Maple)
Acer carpinifolium (Hornbeam Maple) Hornbeam maple is originated from Japan, small tree or large shrub with multi-trunks and with a deciduous leaves resemble to the leaf of Carpinus. The tree is dioecious , male and female flowers are on separate trees. Used as a specimen but rate, will be difficult to locate in commerce. Landscape Information French Name: Erable à feuilles de charme Pronounciation: AY-ser kar-pine-ih-FOH-lee- um Plant Type: Tree Origin: Japan Heat Zones: 1, 2, 3, 4, 5, 6, 7 Hardiness Zones: 4, 5, 6, 7, 8 Uses: Hedge, Topiary, Bonsai, Espalier, Shade Size/Shape Growth Rate: Slow Tree Shape: Round Canopy Symmetry: Symmetrical Plant Image Canopy Density: Medium Canopy Texture: Fine Height at Maturity: 5 to 8 m Spread at Maturity: 5 to 8 meters Time to Ultimate Height: 10 to 20 Years Acer carpinifolium (Hornbeam Maple) Botanical Description Foliage Leaf Arrangement: Opposite Leaf Venation: Pinnate Leaf Persistance: Deciduous Leaf Type: Simple Leaf Blade: 5 - 10 cm Leaf Shape: Ovate Leaf Margins: Serrate Leaf Textures: Medium Leaf Scent: No Fragance Color(growing season): Green Color(changing season): Yellow Flower Flower Showiness: False Flower Scent: No Fragance Flower Color: Green Seasons: Spring Trunk Trunk Susceptibility to Breakage: Generally resists breakage Number of Trunks: Multi-Trunked, Can be trained to one trunk Flower Image Trunk Esthetic Values: Not Showy, Smooth Fruit Fruit Type: Samara Fruit Showiness: False Fruit Size Range: 3 - 7 Fruit Colors: Green, Brown Seasons: Summer, Fall Acer carpinifolium (Hornbeam -

The Evolution and Phylogeny of Beetles
Darwin, Beetles and Phylogenetics Rolf G. Beutel1 . Frank Friedrich1, 2 . Richard A. B. Leschen3 1) Entomology group, Institut für Spezielle Zoologie und Evolutionsbiologie mit Phyletischem Museum, FSU Jena, 07743 Jena; e-mail: [email protected]; 2) Biozentrum Grindel und Zoologisches Museum, Universität Hamburg, 20144 Hamburg; 3) New Zealand Arthropod Collection, Private Bag 92170, Auckland, NZ Whenever I hear of the capture of rare beetles, I feel like an old warhorse at the sound of a trumpet. Charles R. Darwin Abstract Here we review Charles Darwin’s relation to beetles and developments in coleopteran systematics in the last two centuries. Darwin was an enthusiastic beetle collector. He used beetles to illustrate different evolutionary phenomena in his major works, and astonishingly, an entire sub-chapter is dedicated to beetles in “The Descent of Man”. During his voyage on the Beagle, Darwin was impressed by the high diversity of beetles in the tropics and expressed, to his surprise, that the majority of species were small and inconspicuous. Despite his obvious interest in the group he did not get involved in beetle taxonomy and his theoretical work had little immediate impact on beetle classification. The development of taxonomy and classification in the late 19th and earlier 20th centuries was mainly characterised by the exploration of new character systems (e.g., larval features, wing venation). In the mid 20th century Hennig’s new methodology to group lineages by derived characters revolutionised systematics of Coleoptera and other organisms. As envisioned by Darwin and Ernst Haeckel, the new Hennigian approach enabled systematists to establish classifications truly reflecting evolution. -

Aliens: the Invasive Species Bulletin Newsletter of the IUCN/SSC Invasive Species Specialist Group
Aliens: The Invasive Species Bulletin Newsletter of the IUCN/SSC Invasive Species Specialist Group ISSN 1173-5988 Issue Number 31, 2011 Coordinator CONTENTS Piero Genovesi, ISSG Chair, ISPRA Editors Editorial pg. 1 Piero Genovesi and Riccardo Scalera News from the ISSG pg. 2 Assistant Editor ...And other news pg. 4 Anna Alonzi Monitoring and control modalities of a honeybee predator, the Yellow Front Cover Photo legged hornet Vespa velutina The yellow-legged hornet Vespa velutina nigrithorax (Hymenoptera: © Photo by Quentin Rome Vespidae) pg. 7 Improving ant eradications: details of more successes, The following people a global synthesis contributed to this issue and recommendations pg. 16 Shyama Pagad, Carola Warner Introduced reindeer on South Georgia – their impact and management pg. 24 Invasive plant species The newsletter is produced twice a year and in Asian elephant habitats pg. 30 is available in English. To be added to the AlterIAS: a LIFE+ project to curb mailing list, or to download the electronic the introduction of invasive version, visit: ornamental plants in Belgium pg. 36 www.issg.org/newsletter.html#Aliens Investigation of Invasive plant Please direct all submissions and other ed- species in the Caucasus: itorial correspondence to Riccardo Scalera current situation pg. 42 [email protected] The annual cost of invasive species to the British economy quantified pg. 47 Published by Eradication of the non-native ISPRA - Rome, Italy sea squirt Didemnum vexillum Graphics design from Holyhead Harbour, Wales, UK pg. 52 Franco Iozzoli, ISPRA Challenges, needs and future steps Coordination for managing invasive alien species Daria Mazzella, ISPRA - Publishing Section in the Western Balkan Region pg.