Recovery Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Federal Register/Vol. 74, No. 44/Monday, March 9, 2009/Notices
10064 Federal Register / Vol. 74, No. 44 / Monday, March 9, 2009 / Notices Applicant: Copperhead Environmental (Rhus michauxii), bunched arrowhead March 24–26, 2009. The meeting is open Consulting, Inc., Paint Lick, (Sagittaria fasciculata), mountain sweet to the public. The meeting agenda will Kentucky, TE171516 pitcher-plant (Sarracenia jonesii), include reports from the Subcommittees The applicant requests amendment of largeflower skullcap (Scutellaria on Incentives, Legal, Science Tools & existing authorization to add authority montana), blue ridge goldenrod Procedures, and Synthesis, and to capture, handle, and release 31 (Solidago spithamaea), Virginia spirea discussion of the draft species of freshwater mussel for (Spiraea virginiana), Cooley’s Recommendations to the Secretary. presence surveys throughout the species meadowrue (Thalictrum cooleyi), and DATES: The meeting is scheduled for ranges in the eastern United States. running buffalo clover (Trifolium March 24–26, 2009, from 11 a.m. to stoloniferum) to develop and maintain Applicant: South Carolina Parks, 5:30 p.m. on March 24, 8 a.m. to 5:30 germ plasm and propagated specimens Recreation and Tourism, Columbia, p.m. on March 25, and 8 a.m. to 3:30 of plants collected from throughout South Carolina TE207117 p.m. on March 26. North Carolina, South Carolina, The applicant requests authorization ADDRESSES: U.S. Fish and Wildlife Alabama, Georgia, Florida, Tennessee, Service, 4401 N. Fairfax Drive, Rooms to harass, inspect nest cavities, and West Virginia, Virginia, and Maryland. conduct other management activities 200 A & B, Arlington, VA 22203. For Applicant: International Carnivorous with the red-cockaded woodpecker more information, see ‘‘Meeting Plant Society, Pinole, California, (Picoides borealis) throughout South Location Information.’’ TE061005 Carolina. -

Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- BIBLIOGRAPHY
Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- BIBLIOGRAPHY BIBLIOGRAPHY Ackerfield, J., and J. Wen. 2002. A morphometric analysis of Hedera L. (the ivy genus, Araliaceae) and its taxonomic implications. Adansonia 24: 197-212. Adams, P. 1961. Observations on the Sagittaria subulata complex. Rhodora 63: 247-265. Adams, R.M. II, and W.J. Dress. 1982. Nodding Lilium species of eastern North America (Liliaceae). Baileya 21: 165-188. Adams, R.P. 1986. Geographic variation in Juniperus silicicola and J. virginiana of the Southeastern United States: multivariant analyses of morphology and terpenoids. Taxon 35: 31-75. ------. 1995. Revisionary study of Caribbean species of Juniperus (Cupressaceae). Phytologia 78: 134-150. ------, and T. Demeke. 1993. Systematic relationships in Juniperus based on random amplified polymorphic DNAs (RAPDs). Taxon 42: 553-571. Adams, W.P. 1957. A revision of the genus Ascyrum (Hypericaceae). Rhodora 59: 73-95. ------. 1962. Studies in the Guttiferae. I. A synopsis of Hypericum section Myriandra. Contr. Gray Herbarium Harv. 182: 1-51. ------, and N.K.B. Robson. 1961. A re-evaluation of the generic status of Ascyrum and Crookea (Guttiferae). Rhodora 63: 10-16. Adams, W.P. 1973. Clusiaceae of the southeastern United States. J. Elisha Mitchell Sci. Soc. 89: 62-71. Adler, L. 1999. Polygonum perfoliatum (mile-a-minute weed). Chinquapin 7: 4. Aedo, C., J.J. Aldasoro, and C. Navarro. 1998. Taxonomic revision of Geranium sections Batrachioidea and Divaricata (Geraniaceae). Ann. Missouri Bot. Gard. 85: 594-630. Affolter, J.M. 1985. A monograph of the genus Lilaeopsis (Umbelliferae). Systematic Bot. Monographs 6. Ahles, H.E., and A.E. -

Harperella (Ptilimnium Nodosum)
Harperella (Ptilimnium nodosum) Common Name they occur. The stream-side type, which used to be Harperella considered a different species, is generally shorter (20 to 45 cm high), has fewer flowers, and blooms Scientific Name later in the year (July to October) than the pond-side Ptilimnium nodosum type which is 35 to 95 cm high and has flowers from May to June. Status Harperella is listed by the United States Fish and Periodic flooding Wildlife Service as federally Endangered but does of streams where not occur on any federal land, so the ability to harperella grows protect the plant from destruction is limited. in West Virginia usually keeps West Virginia Status large amounts of Harperella is known to grow along three streams in soil from building West Virginia. An additional site was located, but up around the this may be an extension of a previously known site. roots. harperella Harperella is known from less than 20 other sites (all can thrive in in the U.S.). small amounts of soil and in Description shallow water, Harperella is an annual plant with a slender, smooth but this flooding stem and hollow, quill-like leaves. Its flowers are prevents most similar in appearance to those of the common road- other plants from growing in the same places and side plant, Queen Anne's lace. They are small, white, competing with Harperella for resources. Thus, lack and delicate and are arranged in clusters called of flooding or even reduction in flooding could umbels. This plant flowers in May and June. Both decrease harperella's chance for survival. -
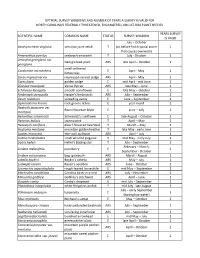
Optimal Survey Windows for At-Risk and Listed Plants of North Carolina
OPTIMAL SURVEY WINDOWS AND NUMBER OF YEARS A SURVEY IS VALID FOR NORTH CAROLINA'S FEDERALLY THREATENED, ENDANGERED, AND AT‐RISK PLANT SPECIES YEARS SURVEY SCIENTIFIC NAME COMMON NAME STATUS SURVEY WINDOW IS VALID July – October Aeschynomene virginica sensitive joint‐vetch T (or before first tropical storm 1 that causes overwash) Amaranthus pumilus seabeach amaranth T July ‐ October 1 Amorpha georgiana var. Georgia lead‐plant ARS late April – October 2 georgiana small‐anthered Cardamine micranthera E April ‐ May 1 bittercress Carex impressinervia impressed‐nerved sedge ARS April ‐ May 2 Carex lutea golden sedge E mid April ‐ mid June 2 Dionaea muscipula Venus flytrap ARS late May – June 2 Echinacea laevigata smooth coneflower E late May – October 2 Fimbristylis perpusilla Harper’s fimbristylis ARS July – September 2 Geum radiatum spreading avens E June – September 2 Gymnoderma lineare rock gnome lichen E year round 2 Hedyotis purpurea var. Roan Mountain bluet E June – July 2 montana Helianthus schweinitzii Schweinitz's sunflower E late August – October 2 Helonias bullata swamp pink T April – May 2 Hexastylis naniflora dwarf‐flowered heartleaf T March – May 2 Hudsonia montana mountain golden heather T late May ‐ early June 2 Isoetes microvela thin‐wall quillwort ARS April – July 1 Isotria medeoloides small whorled pogonia T mid May ‐ early July 1 Liatris helleri Heller's blazing star T July – September 2 February – March; Lindera melissifolia pondberry E 2 September ‐ October Lindera subcoriacea bog spicebush ARS March ‐ August 2 Lobelia -

Federally Protected Plant Species in Georgia Dr
Pub. No. 50 December 2016 Federally Protected Plant Species In Georgia Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia Endangered and threatened species of plants are given federal protection under regulations and agreements stemming from the Endangered Species Act, as amended. Federal protection includes individuals of a listed species, habitats essential for their survival, and specific limitations on pesticide use. This publication lists endangered and threatened plant species by scientific name, common name, federal listing status, and general Georgia county name locations. Figure 1 is a map giving county locations of federally protected plant species in Georgia by identification number. This publication was developed for educational purposes and is not a regulatory document. The State of Georgia (Department of Natural Resources) maintains a com- plete list of both federal and state endangered, threatened, rare and unusual plant species for Georgia. Included at the end of this publication are plant species with a federal status of "candidate," or "in a petition process," for potential listing. Figure 2. Endangered / Threatened Plant Species ID common name #(scientific name) status1 county distribution2 1. Little amphianthus / pool sprite / snorklewort (Amphianthus pusillus) T Granite outcrops in Butts, Columbia, Dekalb, Douglas, Greene, Gwinnett, Hancock, Harris, Heard, Henry, Meriwether, Newton, Oglethorpe, Pike, Putnam, Rockdale, & Walton Dr. Kim D. Coder Warnell School University of Georgia 1 ID common name #(scientific name) status1 county distribution2 2. Georgia rockcress T Chatahoochee, Clay, Harris, (Arabis georgiana) Muscogee, & Stewart 3. hairy rattleweed / cobwebby wild indigo (Baptisia arachnifera) E Brantley & Wayne 4. Alabama leatherflower (Clematis socialis) E Floyd 5. -

Rare Plant Programs
. to inspire understanding, appreciation, and conservation of plants in gardens and Recovering North Carolina’s Rare Plants natural areas and to advance a sustainable ver 40 years ago, the North Carolina Botanical Garden introduced and began Rare Plant relationship between people and nature. Opromoting conservation through propagation—the practice of propagating native plants, for for use in the garden and landscape, via seeds and cuttings as an alternative to —from the mission of the the then widespread practice of wild-collection of native plants. Today, we continue that North Carolina Botanical Garden Programs important work as well as other programs that support the restoration and recovery of our native flora. Other early Garden initiatives included the establishment of a membership support organization, the Botanical Garden Foundation, Inc., to hold conservation lands and support the Garden’s mission (1966); helping establish the Plant Conservation Program in the NC Department of Agriculture and Consumer Services (1979); and becoming a founding member of the national Center for Plant Conservation (1984). Please see the Garden’s web page (www.ncbg.unc.edu), under the About Us tab, for a more complete history and profile of our conservation activities. Rare plant reintroduction TOTAL N.C. STATUS U.S. STATUS and monitoring programs E T SC SR E T C FSC at the Garden include Planting Pickering’s dawnflower (Stylisma reintroduction of Flowering Plants 4,247 109 46 20 529 17 9 5 99 pickeringii var. pickeringii) in a sand barren— Mosses 440 5 1 - 122 - - - 3 harperella (Ptilimnium this rare plant’s typical habitat. Liverworts 225 2 - - 62 - - - 7 nodosum, an Endangered Hornworts 9 - - - 2 - - - 1 On the front of this brochure. -

Coosa & Warrior – Section 7 Meeting – July 22, 2004 Meeting Location
Coosa & Warrior – Section 7 Meeting – July 22, 2004 Meeting Location - APC Corporate Headquarters - Birmingham, AL Revision 08-02-04 Attendees Jim Crew APC Jeff Powell USFWS Patric Harper USFWS Bill Sim APC Stephen Gidiere B&B Rob Fowler B&B Henry Mealing Kleinschmidt Shane Boring Kleinschmidt Action Items Due Date • Distribute copies of the Tapoco BA to the group. Shane Boring August 5, 2004 • Draft meeting notes from 7-22-04 and distribute to attendees for comment Jim Crew August 5, 2004 • Review and Comment on the revised Section 7 Species Tracking Tables USFWS and APC August 1, 2004 • Begin filling in Species Tracking Table and work on BA Shane Boring August 5, 2004 – give update • Prepare for next Section 7 Meeting All Attendees August 5, 2004 @ 10:00 • Continue to investigate USFWS coordination with USFS and NOAA Fisheries Jeff Powell, Patric Harper, or Larry Goldman ASAP Meeting Notes These notes summarize the major items discussed during the meeting and are not intended to be a transcript or analysis of the meeting. Review of Action Items from last meeting: • USFWS response to APC’s January 14, 2004 letters APC has received USFWS Section 7 letters for both the Coosa and Warrior Projects. • Review Draft meeting notes from 02-18-04 Section 7 Meeting APC sent out draft meeting notes on 3-1-04 and we received no comments. • Investigate USFWS coordination with USFS and NOAA Fisheries USFWS is still checking into this subject. Henry began the meeting by stating the meeting goals: 1) Identify and discuss the structure of the Biological Assessment (BA) 2) Identify and discuss an acceptable method to track species information and priorities 3) Identify and discuss the future meeting schedule. -

Survey Periods for Federally Listed Plants and Procedures for Positive Federally Listed Plant Surveys
Survey Periods for Federally Listed Plants and Procedures for Positive Federally Listed Plant Surveys The U.S. Fish and Wildlife Service (Service), West Virginia Field Office (WVFO), has established the following acceptable survey periods for identifying threatened and endangered plants that occur in West Virginia. Surveys conducted during these time frames by approved surveyors should produce reliable results that will be accepted during environmental review. Surveys conducted outside of these periods may allow for identification of suitable habitat, but are not sufficient to confirm that these species are absent within suitable habitat. Negative surveys will be considered valid for two full survey periods from the year of the survey. For example, a survey conducted on July 31, 2012, would be valid through September 30, 2014. If work was to be conducted in a surveyed area after that date, additional surveys may be required. Scientific Name Common Name Status Survey Period Arabis serotina Shale barren rock Endangered August 1 – September cress 30 Isotria medeoloides Small whorled Threatened May 1 – September 30 pogonia Ptilimnium nodosum Harperella Endangered July 1 – September 30* Scirpus Northeastern bulrush Endangered August 1 – September ancistrocheatus 30 Spiraea virginiana Virginia spiraea Threatened June1 – September 30 Trifolium Running buffalo Endangered May 1 – September 30 stoloniferum clover * Surveys should be conducted during periods of low water. To assist in the review process for proposed actions that may affect federally listed threatened and endangered plants, the WVFO, in coordination with the West Virginia Division of Natural Resources (WVDNR), has developed the following procedures if a threatened and endangered plant is found during a survey: 1. -

Ptilimnium Nodosum), Also Known As Bishop’S Weed, Is an Annual Plant from the Carrot Family
TREES AND PLANTS HARPERELLA ABOUT Harperella (Ptilimnium nodosum), also known as Bishop’s Weed, is an annual plant from the Carrot family. It grows 6-36 inches (0.15-1 m) in height and has weak-looking stems with hollow, quill-like leaves. This delicate wildflower is aromatic (scented) and smells like Dill herb. It produces flat clusters of small white flowers at the top of the stems and blooms intermittently from May until first frost. This plant is found in rocky or gravelly shoals of clear, swift-flowing streams and along the edges of shallow, intermittently flooded ponds and wet meadows. Seeds are oval and flat, and although the pollination process has not been studied, seed dispersal seems to be profuse since populations can achieve high density in localized areas, especially along rivers. Harperella tolerates – and may need – a very specific water regime, which includes moderately intensive spring floods. After floodwaters recede, seeds germinate in shallow rocky crevices and complete their life cycle with root systems that are submerged or saturated. DID YOU KNOW? Harperella is a rare and endangered wildflower that is found in only 10 sites in Alabama, Arkansas, Georgia, Maryland, North Carolina, South Carolina, and West Virginia. It was named after Dr. Ronald Harper, who discovered it in 1902. Because Harperella’s life cycle depends on water levels fluctuating at certain times in the growth cycle, it can easily be destroyed by minor disturbances. The greatest threats to its survival are polluting and loss of habitat, silting of water from construction and mining activities, and excessive nutrients entering the streams where it is found. -

Welcome to Virginia, Harperella! ^
A publication of the VIRGINIA NATIVE PLANT SOCIETY Conserving wild flowers and wild places www.vnps.org Welcome to Virginia, Harperella! ^ Harperella (Ptilimnium nodosum), harperella was believed to be an an¬ behaves as a perennial. Some botanists a diminutive herb in the carrot fam¬ nual; it is now known that the type believe that this type, which includes ily, was found for the first time in Vir¬ that occurs along fast-flowing creeks the new Virginia population, is a ginia last June by Virginia Depart¬ and rivers (as opposed to pond edges) (See Harperella, page 4) ment of Conservation and Recreation, Division of Natural Heritage, field botanist Allen Belden. This species is listed as endangered under the federal Harperella Endangered Species Act. The popula¬ Ptilimnium nodosum Illustrations by tion was located along Aquia Creek on Nicky Staunton Marine Corps Base Quantico property in Stafford County. Harperella is unusual in that its leaves, hollow quill-like structures, consist of a leaf stalk (petiole) only; thus, the expanded portion of the leaf (the blade) is missing. The flowers are white and in flat-topped clusters (umbels), resembling those of Queen Anne's lace but much smaller and more delicate. For a long time Exploring the complexities of biodiversity in Richmond Biodiversity, explored from several biodiversity. Some of them are not those indicators of human well-being are not perspectives, was the focus of this we usually hear. He emphasized that based on growth. Discussion brought year's VNPS workshop, held March 8 such a case must include both the val¬ out connections between the two: in Richmond. -

Status Report on Harperella, Ptilimnium Nodosum (Rose) Mathias, in Arkansas Edith L
CORE Metadata, citation and similar papers at core.ac.uk Provided by ScholarWorks@UARK Journal of the Arkansas Academy of Science Volume 55 Article 27 2001 Status Report on Harperella, Ptilimnium nodosum (Rose) Mathias, in Arkansas Edith L. Hardcastle University of Arkansas, Fayetteville David X. Williams University of Arkansas, Fayetteville Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Botany Commons Recommended Citation Hardcastle, Edith L. and Williams, David X. (2001) "Status Report on Harperella, Ptilimnium nodosum (Rose) Mathias, in Arkansas," Journal of the Arkansas Academy of Science: Vol. 55 , Article 27. Available at: http://scholarworks.uark.edu/jaas/vol55/iss1/27 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This General Note is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected]. Journal of the Arkansas Academy of Science, Vol. 55 [2001], Art. 27 A Status Report on Harperella, Ptilimnium nodosum (Rose) Mathias, in Arkansas Edith L.Hardcastle and David X Williams University of Arkansas, Fayetteville, Department of Biological Sciences Fayetteville, Arkansas 72703 Introduction total length of the occurrence is determined and offset lines are established at right angles to the long axis of the A survey of the federally endangered Ptilimnium occurrence. -

Species in Oklahoma
Federally Endangered, Threatened, Proposed, and Candidate Species in Oklahoma SCIENTIFIC NAME COMMON NAME STATUS Birds Vermivora bachmanii Bachman's warbler LE-EX Vireo atricapilla Black-capped vireo LE Numenius borealis Eskimo curlew LE-EX Sterna antillarum Interior least tern LE Campephilus principalis Ivory-billed woodpecker LE-EX Tympanuchus pallidicinctus Lesser prairie chicken PT Charadrius melodus Piping plover LT Anthus spragueii Sprague's pipit C Picoides borealis Red-cockaded woodpecker LE Grus americana Whooping crane LE Fishes Etheostoma cragini Arkansas darter C Notropis girardi Arkansas River shiner LT Percina pantherina Leopard darter LT Noturus placidus Neosho madtom LT Amblyopsis rosae Ozark cavefish LT Insects Nicrophorus americanus American burying beetle LE Mammals Mustela nigripes Black-footed ferret LE-EX Myotis grisescens Gray bat LE Canis lupus Gray wolf LE-EX Ursus arctos horribilis Grizzly bear LT-EX Myotis sodalis Indiana bat LE Corynorhinus townsendii ingens Ozark big-eared bat LE Canis rufus Red wolf LE-EX Mussels Lampsilis rafinesqueana Neosho mucket mussel PE Arkansia wheeleri Ouachita rock-pocketbook mussel LE Quadrula cylindrica cylindrica Rabbitsfoot mussel PT Leptodea leptodon Scaleshell mussel LE Quadrula fragosa Winged mapleleaf mussel LE Reptiles Alligator Mississippiensis American alligator LT-SA Plants Platanthera leucophaea Eatern prairie fringed orchid LT-EX Platanthera praeclara Western prairie fringed orchid LT-EX Ptilimnium nodosum Harperella LE Key LE Listed endangered LT Listed threatened PE Proposed Endangered PT Proposed threatened EX Believed to be extirpated in Oklahoma SA Endangered due to similarity of appearance to other listed species. C Candidate (The Service has enough information to list the species as threatened or endangered, but this action is precluded by other listing activities).