Federal Register/Vol. 85, No. 85/Friday, May 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
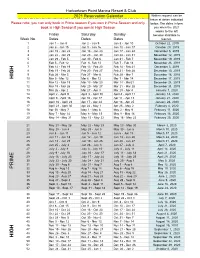
2021 Calandar
Harbortown Point Marina Resort & Club 2021 Reservation Calendar Written request can be taken at dates indicated Please note: you can only book in Prime season if you own in Prime Season and only below. The dates inform book in High Season if you own in High Season you when the 2021 weeks to the left Friday Saturday Sunday become abailable to Week No. Dates Dates Dates reserve. 1 Jan 1 - Jan 8 Jan 2 - Jan 9 Jan 3 - Jan 10 October 22, 2019 2 Jan 8 - Jan 15 Jan 9 - Jan 16 Jan 10 - Jan 17 October 29, 2019 3 Jan 15 - Jan 22 Jan 16 - Jan 23 Jan 17 - Jan 24 November 5, 2019 4 Jan 22 - Jan 29 Jan 23 - Jan 30 Jan 24 - Jan 31 November 12, 2019 5 Jan 29 - Feb 5 Jan 30 - Feb 6 Jan 31 - Feb 7 November 19, 2019 6 Feb 5 - Feb 12 Feb 6- Feb 13 Feb 7 - Feb 14 November 26, 2019 7 Feb 12 - Feb 19 Feb 13 - Feb 20 Feb 14 - Feb 21 December 3, 2019 8 Feb 19 - Feb 26 Feb 20 - Feb 27 Feb 21 - Feb 28 December 10, 2019 9 Feb 26 - Mar 5 Feb 27 - Mar 6 Feb 28 - Mar 7 December 18, 2018 HIGH 10 Mar 5 - Mar 12 Mar 6 - Mar 13 Mar 7 - Mar 14 December 17, 2019 11 Mar 12 - Mar 19 Mar 13 - Mar 20 Mar 14 - Mar21 December 24, 2019 12 Mar 19 - Mar 26 Mar 20 - Mar 27 Mar 21 - Mar 28 December 31, 2019 13 Mar 26 - Apr 2 Mar 27 - Apr 3 Mar 28 - Apr 4 January 7, 2020 14 April 2 - April 9 April 3 - April 10 April 4 - April 11 January 14, 2020 15 April 9 - April 16 Apr 10 - Apr 17 Apr 11 - Apr 18 January 21, 2020 16 April 16 - April 23 Apr 17 - Apr 24 Apr 18 - Apr 25 January 28, 2020 17 April 23 - April 30 Apr 24 - May 1 Apr 25 - May 2 February 4, 2020 18 Apr 30 - May 7 May 1 - May -

Monday, April 5, 2021
Monday, April 5, 2021 WHERE WE ARE TODAY 3 April 5, 2021 OUR PROGRESS ON VACCINATIONS 4 # DC residents partially or fully vaccinated January 1 5,846 February 1 40,839 March 1 74,811 April 1 162,669 (Apr 2) Data source: DC Health, using received data through 4/2/2021. Data subject to change. April 5, 2021 5 We have more work to do to get all residents vaccinated. Make sure you’re signed up to get your COVID-19 shot. Go to: Call: vaccinate.dc.gov 1-855-363-0333 April 5, 2021 PRE-REGISTRATION SNAPSHOT 6 Approximately 190,621 people have pre-registered for a vaccination appointment and are awaiting an appointment. April 5, 2021 7 If you pre-registered for a vaccination appointment but already received your vaccine elsewhere you can email [email protected] to remove yourself from the pre-registration list. April 5, 2021 NEW AND UPCOMING CLINICS 8 This week, the vaccine clinic at the Convention Center will expand through a partnership with Safeway. Appointment invitations will be sent on April 6. On Friday, April 9, a new high-capacity vaccination site will open at Arena Stage. The site will be operated in partnership with DC Health and MedStar Health. Appointments will be made through vaccinate.dc.gov and the District’s call center. April 5, 2021 THE PATH FORWARD FIVE FACTORS DETERMINING AN ACTIVITY’S RE-OPEN POTENTIAL 10 1. MASKS - Can and is the activity completed with or without wearing a mask? Highest Risk Unmasked, Indoors, Long Time, Very Dense 2. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
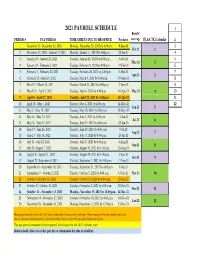
Payroll Calendar 2021
2021 PAYROLL SCHEDULE 1 Benefit PERIOD # PAY PERIOD TIME SHEETS DUE TO HR OFFICE Paydates coverage FLSA 7K Calendar 2 1 December 13- December 26, 2020 Monday, December 28, 2020 by 4:00 p.m. 8-Jan-21 3 Feb-21 1 2 December 27, 2020 - Janurary 9, 2021 Monday, January 11, 2021 by 4:00 p.m. 22-Jan-21 4 3 January 10 - January 23, 2021 Tuesday, January 26, 2021 by 4:00 p.m. 5-Feb-21 5 Mar-21 2 4 January 24 - February 6, 2021 Tuesday, February 9, 2021 by 4:00 p.m. 19-Feb-21 6 5 February 7 - February 20, 2021 Tuesday, February 26, 2021 by 4:00 p.m. 5-Mar-21 7 Apr-21 3 6 February 21 - March 6, 2021 Tuesday, March 9, 2021 by 4:00 p.m. 19-Mar-21 8 7 March 7 - March 20, 2021 Tuesday, March 23, 2021 by 4:00 p.m. 2-Apr-21 9 8 March 21 - April 3, 2021 Tuesday, April 6, 2021 by 4:00 p.m. 16-Apr-21 May-21 4 10 9 April 4 - April 17, 2021 Tuesday, April 20, 2021 by 4:00 p.m. 30-Apr-21 11 10 April 18 - May 1, 2021 Tuesday, May 4, 2021 by 4:00 p.m. 14-May-21 12 Jun-21 5 11 May 2 - May 15, 2021 Tuesday, May 18, 2021 by 4:00 p.m. 28-May-21 12 May 16 - May 29, 2021 Tuesday, June 1, 2021 by 4:00 p.m. 11-Jun-21 Jul-21 6 13 May 30 - June 12, 2021 Tuesday, June 15, 2021 by 4:00 p.m. -

Cuesta Student Planning Calendar
SUMMER SESSION 2021: June 14 – July 23 WINTER BREAK: December 18 - January 17 March 26……….....……...Class Finder available online 2021-2022 December 23–24, 27–30..…..................Board Holidays Beginning April 1………………………….…Apply online STUDENT December 31.……New Year’s Day Holiday (observed) April 19….......……………Priority myCuesta reg begins PLANNING CALENDAR January 12–14, 2022.…..……...……………..Flex Days April 22.………...New/returning/transfer student priority January 17, 2022……….Martin Luther King, Jr Holiday April 27..…............Enrichment/Dual Enrollment priority June 2021 December 2021 May 1...…..…International student application deadline U M T W R F S U M T W R F S SPRING SEMESTER 2022: January 18 – May 20 Day Prior to 1st Class Meeting……..Drop for full refund 1 2 3 4 5 1 2 3 4 6 7 8 9 10 11 12 5 6 7 8 9 10 11 Day Prior to Census Date……..Late add with add code Beginning October 1, 2021…...…......…….Apply online 13 14 15 16 17 18 19 12 13 14 15 16 17 18 October 29..………..…….Class Finder available online July 5 (observed).........……Independence Day Holiday 20 21 22 23 24 25 26 19 20 21 22 23 24 25 27 28 29 30 26 27 28 29 30 31 November 9……..………..Priority myCuesta reg begins July 2021 January 2022 November 22…. New/returning/transfer student priority U M T W R F S U M T W R F S FALL SEMESTER 2021: August 16 – December 17 1 2 3 1 December 1..………International student application deadline 4 5 6 7 8 9 10 2 3 4 5 6 7 8 Beginning October 1, 2020....………..…….Apply online December 3…..…Enrichment/Dual Enrollment student priority 11 12 13 14 15 -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -
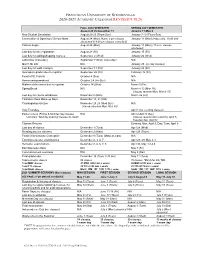
2020-2021 Academic Calendar Revised 9.18.20
FRANCISCAN UNIVERSITY OF STEUBENVILLE 2020-2021 ACADEMIC CALENDAR REVISED 9.18.20 FALL 2020 SEMESTER SPRING 2021 SEMESTER August 24 25-December 11 January 11-May 5 New Student Orientation August 20-23 (Thurs-Sun) January 7-10 (Thurs-Sun) Convocation & Opening of School Mass August 24 (Mon) (4 pm; 3 pm classes January 11 (Mon) (mass only, 10:30 am) shortened & 4:30 pm classes cancelled) Classes begin August 24 (Mon) January 11 (Mon) (10 a.m. classes shortened) Last day for late registration August 28 (Fri) January 15 (Fri) Last day for adding/dropping courses September 2 (Wed) January 20 (Wed) Labor Day (class day) September 7 (Mon) (class day) N/A March for Life N/A January 29 (no day classes) Last day for audit changes September 11 (Fri) January 22 (Fri) Incomplete grades due to registrar September 25 (Fri) February 12 (Fri) Feast of St. Francis October 4 (Sun) N/A Homecoming weekend October 2-4 (Fri-Sun) N/A Midterm deficiencies due to registrar October 14 (Wed) March 5 (Fri) Spring Break N/A March 8-12 (Mon-Fri) (classes resume Mon, March 15) Last day for course withdrawal November 2 (Mon) March 26 (Fri) Tentative Class Make-up Days November 14, 21 (Sat) Thanksgiving vacation November 25-29 (Wed-Sun) N/A (classes resume Mon, Nov 30) Holy Thursday April 1 (no evening classes) Easter recess (Friday & Monday day classes N/A April 2-April 5 (day) canceled; *Monday evening classes do meet) (classes resume Mon evening, April 5, Tuesday day, April 6) Classes Resume Evening: Mon, April 5; Day: Tues, April 6 Last day of classes December 1 (Tues) -
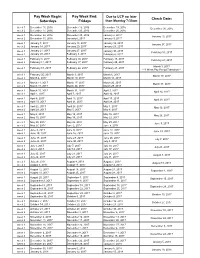
Pay Week Begin: Saturdays Pay Week End: Fridays Check Date
Pay Week Begin: Pay Week End: Due to UCP no later Check Date: Saturdays Fridays than Monday 7:30am week 1 December 10, 2016 December 16, 2016 December 19, 2016 December 30, 2016 week 2 December 17, 2016 December 23, 2016 December 26, 2016 week 1 December 24, 2016 December 30, 2016 January 2, 2017 January 13, 2017 week 2 December 31, 2016 January 6, 2017 January 9, 2017 week 1 January 7, 2017 January 13, 2017 January 16, 2017 January 27, 2017 week 2 January 14, 2017 January 20, 2017 January 23, 2017 January 21, 2017 January 27, 2017 week 1 January 30, 2017 February 10, 2017 week 2 January 28, 2017 February 3, 2017 February 6, 2017 week 1 February 4, 2017 February 10, 2017 February 13, 2017 February 24, 2017 week 2 February 11, 2017 February 17, 2017 February 20, 2017 March 3, 2017 week 1 February 18, 2017 February 24, 2017 February 27, 2017 ***1 Week Pay Period Transition*** week 1 February 25, 2017 March 3, 2017 March 6, 2017 March 17, 2017 week 2 March 4, 2017 March 10, 2017 March 13, 2017 week 1 March 11, 2017 March 17, 2017 March 20, 2017 March 31, 2017 week 2 March 18, 2017 March 24, 2017 March 27, 2017 week 1 March 25, 2017 March 31, 2017 April 3, 2017 April 14, 2017 week 2 April 1, 2017 April 7, 2017 April 10, 2017 week 1 April 8, 2017 April 14, 2017 April 17, 2017 April 28, 2017 week 2 April 15, 2017 April 21, 2017 April 24, 2017 week 1 April 22, 2017 April 28, 2017 May 1, 2017 May 12, 2017 week 2 April 29, 2017 May 5, 2017 May 8, 2017 week 1 May 6, 2017 May 12, 2017 May 15, 2017 May 26, 2017 week 2 May 13, 2017 May 19, 2017 May -

2020-2021 Academic Calendar
The Christ College of Nursing and Health Sciences 2020-2021 College Academic Calendar FALL SEMESTER 2020 16 Weeks Regular Session (FA) First 8 Weeks: Session F1 Second 8 Weeks: Session F2 RN-BSN & HCA Program Orientation opens Online Mon, August 3 Mon, August 3 Mon, August 3 Tuition & Fees Due for Fall Semester Thurs, August 5 Thurs, August 5 Thurs, August 5 ABSN & BSN Nursing Program Orientation – morning and Thurs, August 13 Tues, August 18 n/a afternoon (for Sophomore students registered for nursing classes in the Fall semester) First Day of Classes Mon, August 24 Mon, August 24 Mon, October 19 Labor Day Holiday (College Closed/No Face-to-Face Mon, September 7 Mon, September 7 Mon, September 7 Classes) Last Day to Add a Class Tues, September 1 Fri, August 28 Fri, October 23 Last Day to request course section change Tues, September 1 Fri, August 28 Fri, October 23 Last Day for 100% Refund Tues, September 1 Fri, August 28 Fri, October 23 Last day to Drop Course with No Grade Assigned Tues, September 1 Fri, August 28 Fri, October 23 Enrollment Census Date Tues, September 8 Tues, September 8 Tues, September 8 Last Day for 75% Refund Thurs, September 10 n/a n/a Last Day for 50% Refund Fri, September 11 Wed, September 9 Tues, November 3 Last Day for 25% Refund Fri, September 25 n/a n/a 0% Refund Effective Mon, September 28 Thurs, September 10 Wed, November 4 Last Day to Withdraw with Grade of ‘W’ Tues, October 13 Mon, September 21 Thurs, November 12 Midterm Tues, October 13 Mon, September 21 Thurs, November 12 Updated 02/22/2021 SKM 1 The -

First Revised Page 1
MCImetro ACCESS TRANSMISSION SERVICES LLC Tennessee Tariff No. 4 D/B/A VERIZON ACCESS TRANSMISSION SERVICES Section 10 - First Revised Page 1 10. SPECIAL ARRANGEMENTS RESERVE FOR FUTURE USE D Material that was previously located on this page is now in the Company’s Tariff No. 5 page 90. D Issued: March 27, 2009 Effective: May 1, 2009 Aneitra Jones, Tariff Administrator 5055 North Point Parkway Alpharetta, Georgia 30022 MCImetro ACCESS TRANSMISSION SERVICES LLC Tennessee Tariff No. 4 D/B/A VERIZON ACCESS TRANSMISSION SERVICES Section 10 - First Revised Page 2 10. SPECIAL ARRANGEMENTS RESERVE FOR FUTURE USE D Material that was previously located on this page is now in the Company’s Tariff No. 5 page 91. D Issued: March 27, 2009 Effective: May 1, 2009 Aneitra Jones, Tariff Administrator 5055 North Point Parkway Alpharetta, Georgia 30022 MCImetro ACCESS TRANSMISSION SERVICES LLC Tennessee Tariff No. 4 D/B/A VERIZON ACCESS TRANSMISSION SERVICES Section 10 - First Revised Page 3 10. SPECIAL ARRANGEMENTS RESERVE FOR FUTURE USE D Material that was previously located on this page is now in the Company’s Tariff No. 5 page 91. D Issued: March 27, 2009 Effective: May 1, 2009 Aneitra Jones, Tariff Administrator 5055 North Point Parkway Alpharetta, Georgia 30022 MCImetro ACCESS TRANSMISSION SERVICES LLC Tennessee Tariff No. 4 D/B/A VERIZON ACCESS TRANSMISSION SERVICES Section 10 - First Revised Page 4 10. SPECIAL ARRANGEMENTS RESERVE FOR FUTURE USE M Material that was previously located on this page is now in the Company’s Tariff No. 5 page 55. M Issued: March 27, 2009 Effective: May 1, 2009 Aneitra Jones, Tariff Administrator 5055 North Point Parkway Alpharetta, Georgia 30022 MCImetro ACCESS TRANSMISSION SERVICES LLC Tennessee Tariff No. -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

2021-2022 Official School Calendar
State of Hawaii – Department of Education 2021-2022 OFFICIAL SCHOOL CALENDAR Teachers’ Work Year - 1st Semester: July 28, 2021 - January 3, 2022; 2nd Semester: January 4, 2022 - May 30, 2022 Students’ Work Year - 1st Semester: August 3, 2021 - December 17, 2021; 2nd Semester: January 4, 2022 - May 26, 2022 Student Teacher Su M T W Th F Sa Week Days Days July 2021 18 19 20 21 22 23 24 1st SEMESTER - 89 Student Days (Ends December 17) 1 0 3 25 26 27 28 29 30 31 July 28: Teachers' First Day 2 4 8 August 1 2 3 4 5 6 7 July 28-August 2: Teacher Work Days (no students) 3 9 13 8 9 10 11 12 13 14 August 3: Students' First Day 4 13 17 15 16 17 18 19 20 21 August 20: Statehood Day 5 18 22 22 23 24 25 26 27 28 Q1 6 23 27 September 29 30 31 1 2 3 4 47 days 7 27 31 5 6 7 8 9 10 11 September 6: Labor Day Ends 8 32 36 12 13 14 15 16 17 18 Oct. 8 9 37 41 19 20 21 22 23 24 25 10 42 46 October 26 27 28 29 30 1 2 11 47 51 3 4 5 6 7 8 9 12 10 11 12 13 14 15 16 October 11-15: Fall Break*** 13 52 56 17 18 19 20 21 22 23 14 57 61 24 25 26 27 28 29 30 Q2 15 62 66 November 31 1 2 3 4 5 6 42 days 16 66 70 7 8 9 10 11 12 13 November 11: Veterans Day Ends 17 71 75 14 15 16 17 18 19 20 November 25: Thanksgiving Dec.