COVID-19 Quality Reporting Programs Guidance Memo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(March 1, 1919) We Hereby Declare That Korea Is an Independent
Primary Source Document with Questions (DBQs) DECLARATION OF INDEPENDENCE (MARCH 1, 1919) Introduction The first decade of Japanese colonial rule in Korea was one of harsh repression. In 1919, however, a group of prominent Koreans secretly prepared a Declaration of Independence rejecting Japanese rule and its presumptions and, on March 1, read the document aloud in Seoul’s Pagoda Park. Months of largely peaceful, nationwide demonstrations followed, ultimately involving more than one million Koreans. Japanese authorities responded with force, resulting in thousands of deaths and an even larger number of arrests before the independence movement was put down. In the aftermath, however, Japanese government officials sought to defuse the situation by allowing for a time greater Korean cultural and political expression, though calls for outright political action against colonial rule were still forbidden. The March 1 movement has remained a touchstone for Korean nationalist sentiment up to the present. Document Excerpts with Questions (Longer selection follows this section) From Sources of Korean Tradition, edited by Yŏng‐ho Ch’oe, Peter H. Lee, and Wm. Theodore de Bary, vol. 2 (New York: Columbia University Press, 2000), 337‐339. © 2000 Columbia University Press. Reproduced with the permission of the publisher. All rights reserved. Declaration of Independence (March 1, 1919) We hereby declare that Korea is an independent state and that Korean are a self‑ governing people. We proclaim it to the nations of the world in affirmation of the principle of the equality of all nations, and we proclaim it to our posterity, preserving in perpetuity the right of national survival. We make this declaration on the strength of five thousand years of history as an expression of the devotion and loyalty of twenty million people. -

In Due Course: 2021 Changes to Virginia's Laws
“All laws enacted at a regular session, . excluding a general appropriation law, shall take effect on the first day of July following the adjournment of the session of the General Assembly at which it has been enacted.” Constitution of Virginia, Article IV, Section 13 In Due Course: 2021 Changes to Virginia’s Laws In Due Course is a selection of legislation passed by the 2021 Regular Session and Special Session I of the General Assembly that is likely to affect the daily lives of the citizens of Virginia. The following legislation has been signed by the Governor and for the most part will go into effect on July 1, 2021. The summaries were prepared by the staff of the Division of Legislative Services. Complete information on actions of the 2021 Regular Session and Special Session I is available on the Legislative Information System. Topics Alcoholic Beverage Control Gaming Public Safety Animal Care & Control Health & Health Professions Social Services Civil Procedure Higher Education Special License Plates Corrections Housing Taxation Criminal Offenses Insurance Technology & Innovation Criminal Procedure Labor & Employment Trade & Commerce Domestic Relations Local Government Traffic Infractions Elections Marijuana Transportation Energy Motor Vehicles Voting Firearms Natural Resources Freedom of Information Public Education Alcoholic Beverage Control HB 1845. Alcoholic beverage control; license fee reform; delay; emergency. The law, which became effective on March 11, 2021, delays the effective date of the 2020 alcoholic beverage control license and fee reform from July 1, 2021, to January 1, 2022. During the period of delay and subject to certain requirements, the law allows on-premises wine or beer licensees to sell wine or beer for off-premises consumption and allows such licensees, as well as off-premises wine or beer licensees, to deliver wine or beer that the licensee is authorized to sell without a delivery permit. -

2021 City of Tonganoxie, Kansas: Application and Review Schedule (1 of 4) PUBLIC HEARING APPLICATIONS
2021 City of Tonganoxie, Kansas: Application and Review Schedule (1 of 4) PUBLIC HEARING APPLICATIONS Rezoning, Special Use Permits, Preliminary Plans, Preliminary Plats, Community Unit Plans, Planned Residential Districts (PUD-R), & Zoning Text Amendments The following table lists the submittal and review deadlines and associated scheduled meeting dates for applications that require a public hearing. Note: Preliminary Plat applications are reviewed and approved by the Planning Commission and are not considered by the City Council. Materials for Public Planning Commission Pre-Application Application Submittal Staff Review Revision Submittal City Council 1 Notice of Acceptance 2 Notice and Notification PC Packets Mailed Meeting Date Meeting Deadline Complete Cutoff Considers On: Letters Submitted by: Meeting/Public 8 Weeks Prior to Within 7 Days of Within 14 Days of 14 Days Prior to 7 Days Prior to 2nd Council Meeting 2 Weeks After Pre- Within 21 Days of Public Hearing Planning Commission Receipt of Complete Receipt of Complete Planning Commission Planning Commission After P.C. Action Application Meeting Hearing Notices/Mailing (1st Thursday) Consideration Application Application Consideration Consideration (1st Monday) January 7, 2021 November 12, 2020 November 25, 2020* December 3, 2020 December 17, 2020 December 10, 2020 December 24, 2020 December 31, 2020 January 18, 2021 February 4, 2021 December 10, 2020 December 24, 2020 December 31, 2020 January 14, 2021 January 7, 2021 January 21, 2021 January 28, 2021 March 1, 2021 March 4, 2021 -
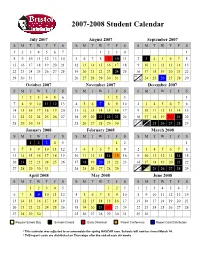
Approved Student Calendar
2007-2008 Student Calendar July 2007 August 2007 September 2007 SMTWT F S SMTWT F S SMTWT F S 1234567 1234 1 8910111213145678910 11 2 3 45678 15 16 17 18 19 20 21 12 13 14 15 16 17 18 9 10 11 12 13 14 15 22 23 24 25 26 27 28 19 20 21 22 23 24 25 16 17 18 19 20 21 22 23 29 30 31 26 27 28 29 30 31 30 24 25 26 27 28 29 October 2007 November 2007 December 2007 SMTWT F S SMTWT F S SMTWT F S 123456 123 1 7891011 12 134567 89102345678 14 15 16 17 18 19 20 11 12 13 14 15 16 17 9 10 11 12 13 14 15 21 22 23 24 25 26 27 18 19 20 21 22 23 24 16 17 18 19 20 21 22 23 24 28 29 30 31 25 26 27 28 29 30 30 31 25 26 27 28 29 January 2008 February 2008 March 2008 SMTWT F S SMTWT F S SMTWT F S 12345 12 1 67891011123456789 2345678 13 14 15 16 17 18 19 10 11 12 13 14 1516 9 1011121314 15 20 21 22 23 24 25 26 17 18 19 20 21 22 23 16 17 18 19 20 21 22 23 24 27 28 29 30 31 24 25 26 27 28 29 30 31 25 26 27 28 29 April 2008 May 2008 June 2008 SMTWT F S SMTWT F S SMTWT F S 12345 123 1234567 6789 10111245678910891011121314 13 14 15 16 17 18 19 11 12 13 14 15 16 17 15 16 17 18 19 20 21 20 21 22 23 24 25 26 18 19 20 21 22 23 24 22 23 24 25 26 27 28 27 28 29 30 25 26 27 28 29 30 31 29 30 Regular School Day Schools Closed Early Dismissal Parent Conference Report Card Distribution * This calendar was adjusted to accommodate the spring NASCAR race. -

Summary of Consolidated Financial Statements for the First Quarter of Fiscal Year Ending December 31, 2021 (IFRS)
Summary of Financial Statements for the First Quarter of Fiscal Year Ending December 31, 2021 Summary of Consolidated Financial Statements for the First Quarter of Fiscal Year Ending December 31, 2021 (IFRS) April 30, 2021 Name of listed company: Nabtesco Corporation Stock listed on: First Section of the Tokyo Stock Exchange Code number: 6268 URL: https://www.nabtesco.com Representative: Title: President and CEO Name: Katsuhiro Teramoto Inquiries: Title: General Manager, Corporate Communication Div. Name: Yasushi Minegishi TEL: +81-3-5213-1134 Scheduled date for filing of quarterly report: May 14, 2021 Scheduled dividend payment date: - Quarterly material to supplement the financial results: Yes Quarterly financial results conference: Yes (Teleconference for institutional investors and financial analysts) (Amounts rounded to the nearest million) 1. Consolidated Results for the First Three-month Period of FY2021 (January 1, 2021 to March 31, 2021) (1) Consolidated Operating Results (Percentages indicate year-on-year change) Net income Income Total comprehensive Net sales Operating income Net income attributable to owners before tax income of the parent Million yen % Million yen % Million yen % Million yen % Million yen % Million yen % First Three-month - - - - Period, FY 2021 72,028 5.0 6,655 (18.7) 124,494 81,115 80,058 84,268 First Three-month Period, FY2020 68,616 (2.4) 8,184 33.0 8,162 10.5 5,575 18.5 5,100 24.9 3,551 (33.9) Basic earnings per share Diluted earnings per share Yen Yen First three-month period, FY2021 647.79 647.75 First three-month period, FY2020 41.09 41.08 (2) Consolidated Financial Position Ratio of equity Equity attributable to Total assets Total equity attributable to owners of owners of the parent the parent Million yen Million yen Million yen % As of March 31, 2021 533,840 280,149 267,740 50.2 As of December 31, 2020 351,723 211,641 198,031 56.3 2. -

Closed Closed
Monday, March 16 Tuesday, March 17 Wednesday, March 18 Thursday, March 19 Friday, March 20 Saturday, March 21 Sunday, March 22 Court 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 8:00 AM Walkers Walkers Walkers Walkers Walkers 8:30 AM 8:00-8:45 AM 8:00-8:45 AM 8:00-8:45 AM 8:00-8:45 AM 8:00-8:45 AM Closed Closed Club Club Club 9:00 AM Club Youth Boys & Girls Boys & Girls Boys & Girls Boys & 9:30 AM Drop-In Pickleball Girls Boys & Drop-In Pickleball Drop-In Pickleball Drop-In Pickleball Drop-In Pickleball Basketball 10:00 AM 9:00-11:00 AM 9:00-11:00 AM 9:00-11:00 AM 9:00-11:00 AM 9:00-11:00 PM Tot Academy Basketball 10:30 AM 10-11 AM 9:00-11:30 AM Rental 11:00 AM 10-1 PM 11:30 AM Drop-In Drop-In Drop-In 12:00 PM Drop-In Pickleball Drop-In Pickleball Adult Basketball Adult Basketball Pickleball 12:30 PM 11:30-1:30 PM 11:30-1:30 PM Rental 11:30-1:30 PM 11:30-1:30 PM 11:30-1:30 PM 1:00 PM 12-2 PM Drop-In Pickleball Drop-In Pickleball 1:30 PM 12:30-2:30 PM 12:30-2:30 PM 2:00 PM Drop-In Boys & Girls Club 2:30 PM Adult/Youth 3:00 PM Drop-In Drop-In Basketball Drop-In Drop-In Adult/Youth Drop-In Adult/Youth 3:30 PM Adult/Youth Adult/Youth 2:00-4:00 PM Adult/Youth Basketball Basketball 4:00 PM Basketball Basketball Tot Basketball 3:00-5:00 PM 3:00-5:00 PM 4:30 PM 3:00-5:00 PM 3:00-5:00 PM Multi- Tumbling Class 3:00-5:00 PM 5:00 PM Sport 4:00-6:00 PM Boys & Girls Club Boys & Girls Club Boys & Girls Club Boys & Girls Club 5:30 PM 4-6 PM 6:00 PM Boy'r Rec Rental Drop-in Drop-in Drop-In Drop-In Drop-in Volleyball 6-7 PM Boy's Rec Drop-In -

2024 7 Day Working Days Calendar
2024 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Monday, January 1, 2024 228 Tuesday, January 2, 2024 229 Wednesday, January 3, 2024 230 Thursday, January 4, 2024 231 Friday, January 5, 2024 232 Saturday, January 6, 2024 233 Sunday, January 7, 2024 234 Monday, January 8, 2024 235 Tuesday, January 9, 2024 236 Wednesday, January 10, 2024 237 Thursday, January 11, 2024 238 Friday, January 12, 2024 239 Saturday, January 13, 2024 240 Sunday, January 14, 2024 241 Monday, January 15, 2024 242 Tuesday, January 16, 2024 243 Wednesday, January 17, 2024 244 Thursday, January 18, 2024 245 Friday, January 19, 2024 246 Saturday, January 20, 2024 247 Sunday, January 21, 2024 248 Monday, January 22, 2024 249 Tuesday, January 23, 2024 250 Wednesday, January 24, 2024 251 Thursday, January 25, 2024 252 Friday, January 26, 2024 253 Saturday, January 27, 2024 254 Sunday, January 28, 2024 255 Monday, January 29, 2024 256 Tuesday, January 30, 2024 257 Wednesday, January 31, 2024 258 Thursday, February 1, 2024 259 Friday, February 2, 2024 260 Saturday, February 3, 2024 261 Sunday, February 4, 2024 262 Date Number of the Calendar Date Monday, February 5, 2024 263 Tuesday, February 6, 2024 264 Wednesday, February -

SJC Third Updated Order Re: Court Operations During COVID-19
COMMONWEALTH OF MASSACHUSETTS SUPREME JUDICIAL COURT SUFFOLK, ss. OE-144 In Re: COVID-19 (Coronavirus) Pandemic THIRD UPDATED ORDER REGARDING COURT OPERATIONS UNDER THE EXIGENT CIRCUMSTANCES CREATED BY THE COVID-19 (CORONAVIRUS) PANDEMIC To safeguard the health and safety of the public and court personnel during the COVID- 19 (coronavirus) pandemic while continuing to increase the business being conducted by the courts, the Supreme Judicial Court (SJC), pursuant to its superintendence and rule-making authority, issues the following ORDER: 1. Prior order. Effective July 1, 2020, this Order shall repeal and replace the Second Updated Order Regarding Court Operations Under The Exigent Circumstances Created By The COVID-19 (Coronavirus) Pandemic, which was issued on May 26, 2020, and took effect on June 1, 2020 (June 1 Order). 2. Conduct of court business and access to courthouses. Courthouses will physically reopen to the public for certain limited purposes on July 13, 2020, as provided in paragraphs 3 through 6. To continue to limit the number of persons entering courthouses, all courts will still conduct most court business virtually (i.e., by telephone, videoconference, email, or comparable means, or through the electronic filing system), in both civil and criminal cases. In cases with one or more self-represented litigants (SRLs) where a court is scheduling a videoconference, courts will recognize the possibility that SRLs may have limited access to the technology needed to conduct videoconferences or limited experience with it, and will either assist the SRL in being able to conduct a videoconference or offer an alternative to videoconferencing for the virtual hearing. -

Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No
Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No. 33 Class & Market Administrator Payment Dates for Producer Milk Component Final Pool Producer Advance Prices Payment Dates Final Payment Due Partial Payment Due Pool Month Prices Release Date Payrolls Due & Pricing Factors PSF, Admin., MS Cooperative Nonmember Cooperative Nonmember January February 3 * February 13 February 22 December 23, 2020 February 16 ** February 16 February 17 Janaury 25 January 26 February March 3 * March 13 March 22 January 21 * March 15 March 16 March 17 February 25 February 26 March March 31 * April 13 April 22 February 18 * April 15 April 16 April 19 ** March 25 March 26 April May 5 May 13 May 22 March 17 * May 17 ** May 17 ** May 17 April 26 ** April 26 May June 3 * June 13 June 22 April 21 * June 15 June 16 June 17 May 25 May 26 June June 30 * July 13 July 22 May 19 * July 15 July 16 July 19 ** June 25 June 28 ** July August 4 * August 13 August 22 June 23 August 16 ** August 16 August 17 July 26 ** July 26 August September 1 * September 13 September 22 July 21 * September 15 September 16 September 17 August 25 August 26 September September 29 * October 13 October 22 August 18 * October 15 October 18 ** October 18 ** September 27 ** September 27 ** October November 3 * November 13 November 22 September 22 * November 15 November 16 November 17 October 25 October 26 November December 1 * December 13 December 22 October 20 * December 15 December 16 December 17 November 26 ** November 26 December January 5, 2022 January 13, 2022 January 22, 2022 November 17 * January 18, 2022 ** January 18, 2022 ** January 18, 2022 ** December 27 ** December 27 ** * If the release date does not fall on the 5th (Class & Component Prices) or 23rd (Advance Prices & Pricing Factors), the most current release preceding will be used in the price calculation. -
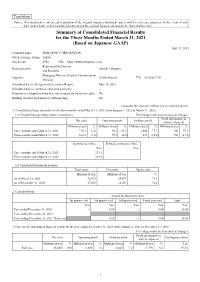
Summary of Consolidated Financial Results for the Three Months Ended
Translation Notice: This document is an excerpt translation of the original Japanese document and is only for reference purposes. In the event of any discrepancy between this translated document and the original Japanese document, the latter shall prevail. Summary of Consolidated Financial Results for the Three Months Ended March 31, 2021 (Based on Japanese GAAP) May 13, 2021 Company name: SEIKO PMC CORPORATION Stock exchange listing: Tokyo Stock code: 4963 URL https://www.seikopmc.co.jp/ Representative Director Representative: Satoshi Takizawa and President Managing Director, Head of Administration Inquiries: Hideki Inouchi TEL 03-6202-7331 Division Scheduled date to file Quarterly Securities Report: May 14, 2021 Scheduled date to commence dividend payments: – Preparation of supplementary material on quarterly financial results: No Holding of quarterly financial results meeting: No (Amounts less than one million yen are rounded down) 1. Consolidated financial results for the three months ended March 31, 2021 (from January 1, 2021 to March 31, 2021) (1) Consolidated operating results (cumulative) Percentages indicate year-on-year changes Profit attributable to Net sales Operating profit Ordinary profit owners of parent Millions of yen % Millions of yen % Millions of yen % Millions of yen % Three months ended March 31, 2021 7,511 13.6 963 62.5 1,054 77.7 700 79.3 Three months ended March 31, 2020 6,613 (1.6) 593 (4.8) 593 (10.6) 390 (21.9) Earnings per share Diluted earnings per share Yen Yen Three months ended March 31, 2021 23.11 – Three months ended March 31, 2020 12.89 – (2) Consolidated financial position Total assets Net assets Equity ratio Millions of yen Millions of yen % As of March 31, 2021 38,972 29,477 71.1 As of December 31, 2020 37,069 28,451 72.4 2. -

Interim Report January 1-March 31, 2021
INTERIM REPORT JANUARY 1-MARCH 31, 2021 “In the first quarter of 2021, net sales declined 22 percent due to lower license sales. Recurring support revenue rose 7 percent and accounts for 38 percent (28) of net sales. Operating profit totaled SEK 12 M (52) and cash flow was SEK 33 M (-4).” Johan Löf, CEO of RaySearch. FIRST QUARTER (JANUARY-MARCH 2021) • Order intake SEK 145.1 M (300.0) • Net sales SEK 162.1 M (208.9) • Operating profit SEK 12.3 M (51.6) • Profit after tax SEK 7.1 M (40.5) • Earnings per share before/after dilution SEK 0.21 (1.18) • Cash flow SEK 32.8 M (-4.2) • Order backlog SEK 1,207.1 M (1,281.5) at the end of the period SIGNIFICANT EVENTS DURING THE FIRST QUARTER • The RayStation® treatment planning system was sold to more leading cancer centers, including Legacy Cancer Institute in the US, the University Hospital in Krakow, Poland, Osaka City University Hospital and Kumamoto University Hospital in Japan. In addition, Inova Schar Cancer Institute in the US and Radiumhospitalet at Oslo University Hospital in Norway expanded their existing RayStation installations. • In March, the Parent Company issued a short-term loan of SEK 200,000 to Vinstandelsstiftelsen RayFoundation on commercial terms. • A wholly owned subsidiary has been established in Australia. THE COVID-19 PANDEMIC • The COVID-19 pandemic with temporary reprioritisations in healthcare had a negative impact on sales in the first quarter. The market situation is gradually improving in line with the vaccine rollout and the recovery is expected to continue. -
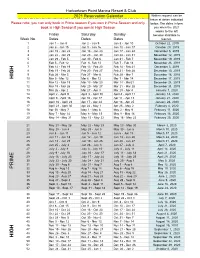
2021 Calandar
Harbortown Point Marina Resort & Club 2021 Reservation Calendar Written request can be taken at dates indicated Please note: you can only book in Prime season if you own in Prime Season and only below. The dates inform book in High Season if you own in High Season you when the 2021 weeks to the left Friday Saturday Sunday become abailable to Week No. Dates Dates Dates reserve. 1 Jan 1 - Jan 8 Jan 2 - Jan 9 Jan 3 - Jan 10 October 22, 2019 2 Jan 8 - Jan 15 Jan 9 - Jan 16 Jan 10 - Jan 17 October 29, 2019 3 Jan 15 - Jan 22 Jan 16 - Jan 23 Jan 17 - Jan 24 November 5, 2019 4 Jan 22 - Jan 29 Jan 23 - Jan 30 Jan 24 - Jan 31 November 12, 2019 5 Jan 29 - Feb 5 Jan 30 - Feb 6 Jan 31 - Feb 7 November 19, 2019 6 Feb 5 - Feb 12 Feb 6- Feb 13 Feb 7 - Feb 14 November 26, 2019 7 Feb 12 - Feb 19 Feb 13 - Feb 20 Feb 14 - Feb 21 December 3, 2019 8 Feb 19 - Feb 26 Feb 20 - Feb 27 Feb 21 - Feb 28 December 10, 2019 9 Feb 26 - Mar 5 Feb 27 - Mar 6 Feb 28 - Mar 7 December 18, 2018 HIGH 10 Mar 5 - Mar 12 Mar 6 - Mar 13 Mar 7 - Mar 14 December 17, 2019 11 Mar 12 - Mar 19 Mar 13 - Mar 20 Mar 14 - Mar21 December 24, 2019 12 Mar 19 - Mar 26 Mar 20 - Mar 27 Mar 21 - Mar 28 December 31, 2019 13 Mar 26 - Apr 2 Mar 27 - Apr 3 Mar 28 - Apr 4 January 7, 2020 14 April 2 - April 9 April 3 - April 10 April 4 - April 11 January 14, 2020 15 April 9 - April 16 Apr 10 - Apr 17 Apr 11 - Apr 18 January 21, 2020 16 April 16 - April 23 Apr 17 - Apr 24 Apr 18 - Apr 25 January 28, 2020 17 April 23 - April 30 Apr 24 - May 1 Apr 25 - May 2 February 4, 2020 18 Apr 30 - May 7 May 1 - May