Dermatology for the Advanced Practice Nurse
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Another Rashmanaging ? Common Skin Problems in Primary Care: Ugh….Another Rash Kathleen Haycraft, DNP, FNP/PNP-BC, DCNP, FAANP Objectives
Another RashManaging ? Common Skin Problems in Primary Care: Ugh….Another Rash Kathleen Haycraft, DNP, FNP/PNP-BC, DCNP, FAANP Objectives At the completion of this session the learner will be able to: 1. Identify common skin rashes seen in dermatology 2. Differentiate between rashes that require urgent treatment and those that require monitored therapy. 3. Determine an appropriate treatment plan for common rashes Financial Disclosures and COI The speaker is on the advisory committee for: ABVIE CELGENE LILLY NOVARTIS PFIZER VALEANT Significance Dermatologic conditions are the number one reason to enter ambulatory walk in clinics The skin it the largest organ of the body and frequently is a measure of what is occurring internally Take a good history Duration What did it look like in the beginning and how has it progressed? Does anyone else in your immediate family or workers have a similar rash? Have you been ill and in what way? What have you treated the rash with prescription or over the counter medications? Take a good history Have they seen anyone and what diagnosis where you given? What is your medical history? What medicines do you take? Does it itch, hurt, scale, or asymptomatic? Give it a scale. How did it begin and what does has it changed (tie this into treatment history)? Is the patient sick? What does it looks like? Macule vs. Patch Papule, nodule, pustule, tumor Vesicle or Bulla Petechial or purpura Indurated vs. non-indurated Is it crusted…deep or superficial What pattern…. Blaschkos vs. dermatome,, symmetrical, central vs. caudal, reticular, annular vs. -

Familial Hyperinsulinism Due to HNF4A Deficiency and Benign Premature Adrenarche: a Case Report
Case Report Familial Hyperinsulinism due to HNF4A Deficiency and Benign Premature Adrenarche: A Case Report Edward Compton,1 David H. Geller,2 Alaina P. Vidmar MD.3 Abstract Background: Familial Hyperinsulinism due to HNF4A deficiency (FHI-HNF4A) is a form of diazoxide-sensitive, diffuse hyperinsulinism, characterized by transient or persistent hyperinsulinemic hypoglycemia, and a propensity to develop Maturity-Onset Diabetes of the Young type 1 (MODY1). The association between FHI-HNF4A deficiency and benign premature adrenarche (BPA) is unknown. The Case: We report the case of a 5-year-old girl with FHI-HNF4A, controlled on diazoxide, who presented with BPA and Tanner stage 3 pubic hair associated with body odor and acne. Work-up revealed elevated dehydroepiandrosterone sulfate (DHEAS), elevated free testosterone, and advanced bone age. Insulin levels were elevated in the setting of normal fasting blood glucose. We discuss the possible hormonal underpinnings of hyperandrogenism. Conclusion: Though the underlying pathophysiology of this phenotype is unclear, a possible synergistic mechanism exists between insulin-induced hyperandrogenism and HNF4A deficiency leading to a transient decrease of SHBG and thus increased free testosterone levels. Further investigation is required to determine the association between HNF4A dysfunction and BPA. Key Words: Hyperinsulinism; Congenital Hyperinsulinism; Adrenarche; HNF4A; Hyperandrogenism (Source: MeSH-NLM). Introduction Congenital hyperinsulinism (CHI) is due to a variety of etiologies that Highlights: result in dysregulated insulin release from pancreatic β-cells. There are Familial Hyperinsulinism due to HNF4A deficiency (FHI-HNF4A) is a two histological variants of CHI, focal and diffuse, which differ in the form of diazoxide-sensitive; diffuse hyperinsulinism, characterized by extent of pancreatic involvement. -
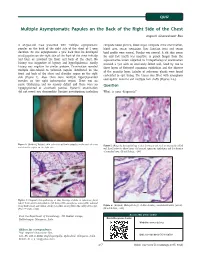
Multiple Asymptomatic Papules on the Back of the Right Side of the Chest Angoori Gnaneshwar Rao
QUIZ Multiple Asymptomatic Papules on the Back of the Right Side of the Chest Angoori Gnaneshwar Rao A 43-year-old male presented with multiple asymptomatic complete blood picture, blood sugar, complete urine examination, papules on the back of the right side of the chest of 1 year blood urea, serum creatinine, liver function tests and serum duration. He was asymptomatic a year back then he developed lipid profile were normal. Fundus was normal. A slit skin smear small papules on the right side of the front of the chest initially for acid fast bacilli was negative. A punch biopsy from the and later on involved the front and back of the chest. No representative lesion subjected to histopathological examination history was suggestive of leprosy and hyperlipidemias. Family revealed a cyst with an intricately folded wall, lined by two to history was negative for similar problem. Examination revealed three layers of flattened squamous epithelium and the absence multiple skin-colored to yellowish papules distributed on the of the granular layer. Lobules of sebaceous glands were found front and back of the chest and shoulder region on the right embedded in cyst lining. The lumen was filled with amorphous side [Figure 1]. Also, there were multiple hyperpigmented eosinophilic material and multiple hair shafts [Figures 2-4]. macules on the right infrascapular region. There was no nerve thickening and no sensory deficit and there were no Question hypopigmented or anesthetic patches. Systemic examination did not reveal any abnormality. Routine investigations including What is your diagnosis? (Original) Multiple skin-colored to yellowish papules on the back of chest Figure 1: Figure 2: (Original) Histopathology of skin showing a cyst with an intricately folded and shoulder region on the right side wall lined by two to three layers of flattened squamous epithelium and the absence of granular layer. -

(ERYTHEMA NODOSUM LEPROSUM) the Term
? THE HISTOPATHOLOGY OF ACUTE PANNICULITIS NODOSA LEPROSA (ERYTHEMA NODOSUM LEPROSUM) w. J. PEPLER, M.B., Ch.B. Department of Pathology, South African Institute for Medical Research, Johannesburg R. KOOIJ, M.D. Westfort Institution, Pretoria AND J. MARSHALL, M.D. University of Pretoria, Pretoria The term "erythema nodosum leprosum" has frequently appeared in the literature on leprosy since the 1930's, apparently popularized by the Japanese (9). The occurrence of "erythema nodosum" in leprosy had previously been noted at the end of the nineteenth century by Brocq (6) and by Hansen and Looft (8), but the term seems to have fallen into disuse. As will become clear from our clinical and histological descriptions of the phenomenon commonly known as erythema nodosum leprosum, we believe the title to be a misnomer. We suggest that it would be more accurate to call it panniculitis nodosa leprosa (P.N.L,). This condition is commonly confused with acute lepra reaction, both processes being referred to as "acute reactions"; but a sharp line of distinction must be drawn between them. P.N.L. occurs only in patients with lepromatous leprosy as an acute, subacute or chronic skin eruption, with or without fever. It is characterized by showers of dusky red nodules, 0.5 to 2 cm. in diameter, on the extensor surfaces of the limbs, on the face, and, less often, on the trunk. The number of lesions appearing in an attack varies from a few to several hundreds, and they sometimes coalesce to form plaques. The nodules may be painful, and they are usually tender to touch. -

Chapter 3 Bacterial and Viral Infections
GBB03 10/4/06 12:20 PM Page 19 Chapter 3 Bacterial and viral infections A mighty creature is the germ gain entry into the skin via minor abrasions, or fis- Though smaller than the pachyderm sures between the toes associated with tinea pedis, His customary dwelling place and leg ulcers provide a portal of entry in many Is deep within the human race cases. A frequent predisposing factor is oedema of His childish pride he often pleases the legs, and cellulitis is a common condition in By giving people strange diseases elderly people, who often suffer from leg oedema Do you, my poppet, feel infirm? of cardiac, venous or lymphatic origin. You probably contain a germ The affected area becomes red, hot and swollen (Ogden Nash, The Germ) (Fig. 3.1), and blister formation and areas of skin necrosis may occur. The patient is pyrexial and feels unwell. Rigors may occur and, in elderly Bacterial infections people, a toxic confusional state. In presumed streptococcal cellulitis, penicillin is Streptococcal infection the treatment of choice, initially given as ben- zylpenicillin intravenously. If the leg is affected, Cellulitis bed rest is an important aspect of treatment. Where Cellulitis is a bacterial infection of subcutaneous there is extensive tissue necrosis, surgical debride- tissues that, in immunologically normal individu- ment may be necessary. als, is usually caused by Streptococcus pyogenes. A particularly severe, deep form of cellulitis, in- ‘Erysipelas’ is a term applied to superficial volving fascia and muscles, is known as ‘necrotiz- streptococcal cellulitis that has a well-demarcated ing fasciitis’. This disorder achieved notoriety a few edge. -

Comparison of Efficacy of Combination of 2% Ketoconazole
Open Access Original Article Comparison of Topical Treatments in Pityriasis Versicolor Pak Armed Forces Med J 2018; 68 (6): 1725-30 COMPARISON OF EFFICACY OF COMBINATION OF 2% KETOCONAZOLE SOLUTION WASH AND TOPICAL 1% CLOTRIMAZOLE WITH TOPICAL 1% CLOTRIMAZOLE ALONE IN CASES OF PITYRIASIS VERSICOLOR Ayesha Anwar, Naeem Raza, Najia Ahmed, Hyder Ali Awan* Pak Emirates Military Hospital/National University of Medical Sciences (NUMS) Rawalpindi Pakistan, *King Abdul Aziz Naval Base, Jubail, Saudia Arabia ABSTRACT Objective: Comparison of efficacy of combination comprising 2% ketoconazole solution wash plus topical 1% clotrimazole versus topical 1% clotrimazole alone in management of patients with Pityriasis versicolor. Study Design: Randomized controlled trial. Place and Duration of Study: Dermatology department, Pak Emirates Military Hospital Rawalpindi, from Oct 2016 to Apr 2017. Material and Methods: Sixty patients of Pityriasis versicolor, both male and female were included in study. Diagnosis of Pityriasis versicolor was made clinically and confirmed microscopically by examining skin scrapings for fungal hyphae. Patients with concomitant systemic illnesses or those who had received anti-fungal in last three months were excluded from study. Random number tables were used to allocate patients to the two treatment groups. Group A received 2% ketoconazole shampoo twice per week for four weeks plus topical 1% clotrimazole twice daily application for 2 weeks. Group B received only topical therapy with 1% clotrimazole cream applied twice daily for 2 weeks. Assessment of treatment efficacy was done by clinical examination of patient and microscopy of skin scrapping for fungal hyphaedone at baseline and at end of study (4 weeks of treatment). A negative clinical examination and negative skin scrapping for fungal hyphae was considered effective therapeutic response. -

The Management of Common Skin Conditions in General Practice
Management of Common Skin Conditions In General Practice including the “red rash made easy” © Arroll, Fishman & Oakley, Department of General Practice and Primary Health Care University of Auckland, Tamaki Campus Reviewed by Hon A/Prof Amanda Oakley - 2019 http://www.dermnetnz.org Management of Common Skin Conditions In General Practice Contents Page Derm Map 3 Classic location: infants & children 4 Classic location: adults 5 Dermatology terminology 6 Common red rashes 7 Other common skin conditions 12 Common viral infections 14 Common bacterial infections 16 Common fungal infections 17 Arthropods 19 Eczema/dermatitis 20 Benign skin lesions 23 Skin cancers 26 Emergency dermatology 28 Clinical diagnosis of melanoma 31 Principles of diagnosis and treatment 32 Principles of treatment of eczema 33 Treatment sequence for psoriasis 34 Topical corticosteroids 35 Combination topical steroid + antimicrobial 36 Safety with topical corticosteroids 36 Emollients 37 Antipruritics 38 For further information, refer to: http://www.dermnetnz.org And http://www.derm-master.com 2 © Arroll, Fishman & Oakley, Department of General Practice and Primary Health Care, University of Auckland, Tamaki Campus. Management of Common Skin Conditions In General Practice DERM MAP Start Is the patient sick ? Yes Rash could be an infection or a drug eruption? No Insect Bites – Crop of grouped papules with a central blister or scab. Is the patient in pain or the rash Yes Infection: cellulitis / erysipelas, impetigo, boil is swelling, oozing or crusting? / folliculitis, herpes simplex / zoster. Urticaria – Smooth skin surface with weals that evolve in minutes to hours. No Is the rash in a classic location? Yes See our classic location chart . -

Autoinvolutive Photoexacerbated Tinea Corporis Mimicking a Subacute Cutaneous Lupus Erythematosus
Letters to the Editor 141 low-potency steroids had no eŒect. Our patient was treated 4. Jarrat M, Ramsdell W. Infantile acropustulosis. Arch Dermatol with a modern glucocorticoid which has an improved risk– 1979; 115: 834–836. bene t ratio. The antipruritic and anti-in ammatory properties 5. Kahn G, Rywlin AM. Acropustulosis of infancy. Arch Dermatol of the steroid were increased by applying it in combination 1979; 115: 831–833. 6. Newton JA, Salisbury J, Marsden A, McGibbon DH. with a wet-wrap technique, which has already been shown to Acropustulosis of infancy. Br J Dermatol 1986; 115: 735–739. be extremely helpful in cases of acute exacerbations of atopic 7. Mancini AJ, Frieden IJ, Praller AS. Infantile acropustulosis eczema in combination with (3) or even without topical revisited: history of scabies and response to topical corticosteroids. steroids (8). Pediatr Dermatol 1998; 15: 337–341. 8. Abeck D, Brockow K, Mempel M, Fesq H, Ring J. Treatment of acute exacerbated atopic eczema with emollient-antiseptic prepara- tions using ‘‘wet-wrap’’ (‘‘wet-pyjama’’) technique. Hautarzt 1999; REFERENCES 50: 418–421. 1. Vignon-Pennam en M-D, Wallach D. Infantile acropustulosis. Arch Dermatol 1986; 122: 1155–1160. Accepted November 24, 2000. 2. Duvanel T, Harms M. Infantile Akropustulose. Hautarzt 1988; 39: 1–4. Markus Braun-Falco, Silke Stachowitz, Christina Schnopp, Johannes 3. Oranje AP, Wolkerstorfer A, de Waard-van der Spek FB. Treatment Ring and Dietrich Abeck of erythrodermic atopic dermatitis with ‘‘wet-wrap’’ uticasone Klinik und Poliklinik fu¨r Dermatologie und Allergologie am propionate 0,05% cream/emollient 1:1 dressing. -

EPA's Guidance to Protect POTW Workers from Toxic and Reactive
United States Office Of Water EPA 812-B-92-001 Environmental Protection (EN-336) NTIS No. PB92-173-236 Agency June 1992 EPA Guidance To Protect POTW Workers From Toxic And Reactive Gases And Vapors DISCLAIMER: This is a guidance document only. Compliance with these procedures cannot guarantee worker safety in all cases. Each POTW must assess whether measures more protective of worker health are necessary at each facility. Confined-spaceentry, worker right-to-know, and worker health and safety issues not directly related to toxic or reactive discharges to POTWs are beyond the scope of this guidance document and are not addressed. Additional copies of this document and other EPA documentsreferenced in this document can be obtained by writing to the National Technical Information Service (NTIS) at: 5285 Port Royal Rd. Springfield, VA 22161 Ph #: 703-487-4650 (NTIS charges a fee for each document.) FOREWORD In 1978, EPA promulgated the General Pretreatment Regulations [40 CFR Part 403] to control industrial discharges to POTWs that damage the collection system, interfere with treatment plant operations, limit sewage sludge disposal options, or pass through inadequately treated into receiving waters On July 24, 1990, EPA amended the General Pretreatment Regulations to respond to the findings and recommendations of the Report to Congress onthe Discharge of Hazardous Wastesto Publicly Owned Treatment Works (the “Domestic Sewage Study”), which identified ways to strengthen the control of hazardous wastes discharged to POTWs. The amendments add -

2U11/13U195 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date Χ n n 20 October 2011 (20.10.2011) 2U11/13U195 Al (51) International Patent Classification: ka Pharmaceutical Co., Ltd., 1-7-1, Dosho-machi, Chuo- C12P 19/34 (2006.01) C07H 21/04 (2006.01) ku, Osaka-shi, Osaka 541-0045 (JP). (21) International Application Number: (74) Agents: KELLOGG, Rosemary et al; Swanson & PCT/US201 1/032017 Bratschun, L.L.C., 8210 SouthPark Terrace, Littleton, Colorado 80120 (US). (22) International Filing Date: 12 April 201 1 (12.04.201 1) (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, English (25) Filing Language: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (26) Publication Language: English CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (30) Priority Data: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 61/323,145 12 April 2010 (12.04.2010) US KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (71) Applicants (for all designated States except US): SOMA- ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, LOGIC, INC. [US/US]; 2945 Wilderness Place, Boulder, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, Colorado 80301 (US). OTSUKA PHARMACEUTI¬ SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, CAL CO., LTD. -

Treatment of Hyperhidrosis Dr
“ Finding a solution to my sweating problem has With advanced technology and skilled hands, wholly changed my life. After having the Botox Matthew R. Kelleher, MD provides a full Premier Dermatology spectrum of services and procedures, including: for hyperhidrosis treatment, I am a thousand • Liposculpture times more confi dent and no longer afraid to • Botox, Juvéderm®, and Voluma™ Treatment TREATMENT OF lift my arms and be completely myself. I am so of Wrinkles thankful that this treatment exists!” • Laser Removal of Age Spots and Freckles HYPERHIDROSIS • Laser Facial Rejuvenation - Olivia • Laser Hair Removal Botox for hyperhidrosis patient • Laser Treatments of Rosacea, Facial Redness, and Spider Veins • Laser Scar Reduction • Laser Treatment of Stretch Marks “ Suffering from axillary hyperhidrosis, I thought • Laser Tattoo Removal • Laser Removal of Vascular Birthmarks there was nothing I could do. My condition • Laser and Photodynamic Treatment of Acne made me reluctant to participate in any social • Sclerotherapy for Leg Veins environment. Every day was a struggle until • Thermage® Radiofrequency Tissue Tightening liposuction for hyperhidrosis changed my life! • Microdermabrasion • Botox and Liposculpture Treatment of Hyperhidrosis Dr. Kelleher gave me the confi dence to feel • Sculpsure and Kybella for nonsurgical body sculpting comfortable in my own skin, and I never have to worry about embarrassing sweat stains again!” - Matthew Liposculpture for hyperhidrosis of the underarms patient “ After dealing with my excessive sweating for many years, without fully understanding it was a medical condition, Dr. Kelleher took the time to explain the treatment options available along with their results. I experienced immediate, positive results after my fi rst treatment which gave me a new sense of confi dence and removed the insurmountable stress I carried daily. -

Material Safety Data Sheet Is for Carbon Dioxide Supplied in Cylinders with 33 Cubic Feet (935 Liters) Or Less Gas Capacity (DOT - 39 Cylinders)
MATERIAL SAFETY DATA SHEET Prepared to U.S. OSHA, CMA, ANSI and Canadian WHMIS Standards 1. PRODUCT AND COMPANY INFORMATION CHEMICAL NAME; CLASS: CARBON DIOXIDE SYNONYMS: Carbon Anhydride, Carbonic Acid Gas, Carbonic Anhydride, Carbon Dioxide USP CHEMICAL FAMILY NAME: Acid Anhydride FORMULA: CO2 Document Number: 50007 Note: This Material Safety Data Sheet is for Carbon Dioxide supplied in cylinders with 33 cubic feet (935 liters) or less gas capacity (DOT - 39 cylinders). For Carbon Dioxide in large cylinders refer to Document Number 10039. PRODUCT USE: Calibration of Monitoring and Research Equipment MANUFACTURED/SUPPLIED FOR: ADDRESS: 821 Chesapeake Drive Cambridge, MD 21613 EMERGENCY PHONE: CHEMTREC: 1-800-424-9300 BUSINESS PHONE: 1-410-228-6400 General MSDS Information 1-713/868-0440 Fax on Demand: 1-800/231-1366 CARBON DIOXIDE - CO2 MSDS EFFECTIVE DATE: AUGUST 31, 2005 PAGE 1 OF 9 2. HAZARD IDENTIFICATION EMERGENCY OVERVIEW: Carbon Dioxide is a colorless, odorless, non-flammable gas. Over-exposure to Carbon Dioxide can increase respiration and heart rate, possibly resulting in circulatory insufficiency, which may lead to coma and death. At concentrations between 2-10%, Carbon Dioxide can cause nausea, dizziness, headache, mental confusion, increased blood pressure and respiratory rate. Exposure to Carbon Dioxide can also cause asphyxiation, through displacement of oxygen. If the gas concentration reaches 10% or more, suffocation can occur within minutes. Moisture in the air could lead to the formation of carbonic acid which can be irritating to the eyes. SYMPTOMS OF OVER-EXPOSURE BY ROUTE OF EXPOSURE: The most significant routes of over-exposure for this gas are by inhalation, and contact with the cryogenic liquid.