Thesis Draft Rough
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wild Bees of Grand Staircase-Escalante National Monument: Richness, Abundance, and Spatio-Temporal Beta-Diversity
Wild bees of Grand Staircase-Escalante National Monument: richness, abundance, and spatio-temporal beta-diversity Olivia Messinger Carril1, Terry Griswold2, James Haefner3 and Joseph S. Wilson4 1 Santa Fe, NM, United States of America 2 USDA-ARS Pollinating Insects Research Unit, Logan, UT, United States of America 3 Biology Department, Emeritus Professor, Utah State University, Logan, UT, United States of America 4 Department of Biology, Utah State University - Tooele, Tooele, UT, United States of America ABSTRACT Interest in bees has grown dramatically in recent years in light of several studies that have reported widespread declines in bees and other pollinators. Investigating declines in wild bees can be difficult, however, due to the lack of faunal surveys that provide baseline data of bee richness and diversity. Protected lands such as national monuments and national parks can provide unique opportunities to learn about and monitor bee populations dynamics in a natural setting because the opportunity for large-scale changes to the landscape are reduced compared to unprotected lands. Here we report on a 4-year study of bees in Grand Staircase-Escalante National Monument (GSENM), found in southern Utah, USA. Using opportunistic collecting and a series of standardized plots, we collected bees throughout the six-month flowering season for four consecutive years. In total, 660 bee species are now known from the area, across 55 genera, and including 49 new species. Two genera not previously known to occur in the state of Utah were discovered, as well as 16 new species records for the state. Bees include ground-nesters, cavity- and twig-nesters, cleptoparasites, narrow specialists, generalists, solitary, and social species. -
Native Plants for Lazy Gardeners - Plant List (10/23/10)
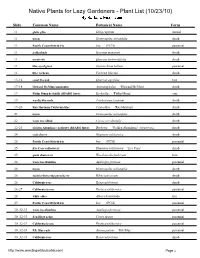
Native Plants for Lazy Gardeners - Plant List (10/23/10) Slide Common Name Botanical Name Form 11 globe gilia Gilia capitata annual 11 toyon Heteromeles arbutifolia shrub 11 Pacific Coast Hybrid iris Iris (PCH) perennial 11 goldenbush Isocoma menziesii shrub 11 scrub oak Quercus berberidifolia shrub 11 blue-eyed grass Sisyrinchium bellum perennial 11 lilac verbena Verbena lilacina shrub 13-16 coast live oak Quercus agrifolia tree 17-18 Howard McMinn man anita Arctostaphylos 'Howard McMinn' shrub 19 Philip Mun keckiella (RSABG Intro) Keckiella 'Philip Munz' ine 19 woolly bluecurls Trichostema lanatum shrub 19-20 Ray Hartman California lilac Ceanothus 'Ray Hartman' shrub 21 toyon Heteromeles arbutifolia shrub 22 western redbud Cercis occidentalis shrub 22-23 Golden Abundance barberry (RSABG Intro) Berberis 'Golden Abundance' (MAHONIA) shrub 2, coffeeberry Rhamnus californica shrub 25 Pacific Coast Hybrid iris Iris (PCH) perennial 25 Eve Case coffeeberry Rhamnus californica '. e Case' shrub 25 giant chain fern Woodwardia fimbriata fern 26 western columbine Aquilegia formosa perennial 26 toyon Heteromeles arbutifolia shrub 26 fuchsia-flowering gooseberry Ribes speciosum shrub 26 California rose Rosa californica shrub 26-27 California fescue Festuca californica perennial 28 white alder Alnus rhombifolia tree 29 Pacific Coast Hybrid iris Iris (PCH) perennial 30 032-33 western columbine Aquilegia formosa perennial 30 032-33 San Diego sedge Carex spissa perennial 30 032-33 California fescue Festuca californica perennial 30 032-33 Elk Blue rush Juncus patens '.l1 2lue' perennial 30 032-33 California rose Rosa californica shrub http://www weedingwildsuburbia com/ Page 1 30 032-3, toyon Heteromeles arbutifolia shrub 30 032-3, fuchsia-flowering gooseberry Ribes speciosum shrub 30 032-3, Claremont pink-flowering currant (RSA Intro) Ribes sanguineum ar. -

Order Hymenoptera, Family Gasteruptiidae
Arthropod fauna of the UAE, 6: 190–224 (2017) Order Hymenoptera, family Gasteruptiidae Christoph Saure, Christian Schmid-Egger & Cornelis van Achterberg INTRODUCTION The family Gasteruptiidae is a small group of wasps comprising about 500 described species in two subfamilies, Gasteruptiinae (four genera) (Macedo, 2011; Zhao et al., 2012) and Hyptiogastrinae (two genera) (Jennings & Austin, 2002). They are easily recognized from other Hymenoptera Apocrita by the elongated ’neck‘ (propleuron), the swollen hind tibiae and the elongate metasoma attached at a high point on the thorax. Adults are free-living insects feeding on nectar mainly on flowers with easily accessible nectar such are of the families Apiaceae, Asteraceae or Euphorbiaceae, but probably at least some Gasteruption species feed on both nectar and pollen (Jennings & Austin, 2004). Gasteruptiidae are also known by their hovering flight during inspection of bee nests (van Achterberg, 2013). The larvae feed on the larval food of solitary bees, after consuming the egg or larva of the bee (Malyshev, 1964, 1965). They select solitary bees of the families Apidae, Colletidae, Halictidae and Megachilidae nesting in stems or in wood, and less often in clay banks or other vertical soil substrates (Zhao et al., 2012; van Achterberg, 2013); as far as known, bees nesting in flat ground are far less often attacked. In Australia, members of the Hyptiogastrinae attend bee nests in the ground (Houston, 1987). There is only indirect evidence that Gasteruptiinae attack wasp nests, e.g. Crabronidae, Sphecidae and solitary Vespidae (Eumeninae) (Jennings & Austin, 2004; van Achterberg, 2013). The pupa of Gasteruptiidae hibernates until the next spring or summer (He, 2004; Jennings & Austin, 2004). -

Description of Mature Larvae and Ecological Notes on Gasteruption Latreille
JHR 65: 1–21 (2018)Description of mature larvae and ecological notes on Gasteruption Latreille... 1 doi: 10.3897/jhr.65.26645 RESEARCH ARTICLE http://jhr.pensoft.net Description of mature larvae and ecological notes on Gasteruption Latreille (Hymenoptera, Evanioidea, Gasteruptiidae) parasitizing hymenopterans nesting in reed galls Petr Bogusch1, Cornelis van Achterberg2, Karel Šilhán1, Alena Astapenková1, Petr Heneberg3 1 Department of Biology, Faculty of Science, University of Hradec Králové, Rokitanského 62, CZ-500 03 Hra- dec Králové, Czech Republic 2 Department of Terrestrial Zoology, Naturalis Biodiversity Center, Pesthuislaan 7, 2333 BA Leiden, The Netherlands 3 Third Faculty of Medicine, Charles University, Ruská 87, CZ-100 00 Praha, Czech Republic Corresponding author: Petr Bogusch ([email protected]) Academic editor: M. Ohl | Received 13 May 2018 | Accepted 19 June 2018 | Published 27 August 2018 http://zoobank.org/D49D4029-A7DA-4631-960D-4B4D7F512B8D Citation: Bogusch P, van Achterberg C, Šilhán K, Astapenková A, Heneberg P (2018) Description of mature larvae and ecological notes on Gasteruption Latreille (Hymenoptera, Evanioidea, Gasteruptiidae) parasitizing hymenopterans nesting in reed galls. Journal of Hymenoptera Research 65: 1–21. https://doi.org/10.3897/jhr.65.26645 Abstract Wasps of the genus Gasteruption are predator-inquilines of bees nesting in cavities in wood, stems, galls, and vertical soil surfaces. During studies of hymenopterans associated with reed galls caused by flies of the genus Lipara we recorded three species. We provide the evidence that a rare European species Gasteruption phragmiticola is a specialized predator-inquiline of an equally rare wetland bee Hylaeus pectoralis. Gasteruption nigrescens is a predator-inquiline of bees of the family Megachilidae, using the common bee Hoplitis leucomelana as the main host. -

Male and Female Bees Show Large Differences in Floral Preference
bioRxiv preprint doi: https://doi.org/10.1101/432518; this version posted November 16, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Male and female bees show large differences in floral preference 2 3 Michael Roswell [email protected] 4 Graduate program in ecology and evolution, Rutgers University 5 14 College Farm Road, New Brunswick, NJ 08904 6 7 Jonathan Dushoff 8 Department of biology, McMaster University 9 1280 Main St. West, Hamilton, Ontario ON L8S 4K1 10 11 Rachael Winfree 12 Department of ecology, evolution, and natural resources, Rutgers University 13 14 College Farm Road, New Brunswick, NJ 08904 1 bioRxiv preprint doi: https://doi.org/10.1101/432518; this version posted November 16, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 14 Abstract 15 16 1. Intraspecific variation in foraging niche can drive food web dynamics and 17 ecosystem processes. Field studies and theoretical analysis of plant-pollinator 18 interaction networks typically focus on the partitioning of the floral community 19 between pollinator species, with little attention paid to intraspecific variation 20 among plants or foraging bees. In other systems, male and female animals 21 exhibit different, cascading, impacts on interaction partners. -

© 2020 Theodore Payne Foundation for Wild Flowers & Native Plants. No
April 24, 2020 Theodore Payne Foundation’s Wild Flower Hotline is made possible by donations, memberships and sponsors. You can support TPF by shopping the online gift store as well. A new, pay by phone, contactless plant pickup system is now available. Details here. Widespread closures remain in place. If you find an accessible trail, please practice social distancing precautions. The purpose for the Wild Flower Hotline now is NOT to send you out to localities to view wild flowers, but to post photos that assure you—virtually—that California’s wild spaces are still open for business for flowers and their pollinators. This week Mother Nature turned on the furnace and with the hot temperatures, our spring love fest with the beloved California poppy will soon come to an end. Throughout the state, poppies are setting both seed and promise for a glorious Spring 2021. Antelope Valley and the surrounding area has a great display of luminous orange California poppies (Eschscholzia californica), electric yellow monolopia (Monolopia lanceolata) and patches of goldfields (Lasthenia sp.). Spotted among the overwhelming yellow-orange color, are lupine (Lupinus spp.), tansy leafed phacelia (Phacelia tanacetifolia) and popcorn flower (Cryptantha spp.). You do not need to leave your home to see the poppies at the Antelope Valley State Poppy Reserve. Just view the live stream online at the preserve via the PoppyCam. Poppies (Eschscholzia californica) in Antelope Valley. Photo by Don Vogt © 2020 Theodore Payne Foundation for Wild Flowers & Native Plants. No reproduction of any kind without written permission. Native plants are blooming and abundant in a South Pasadena nature park. -

Apoidea (Insecta: Hymenoptera). Fauna of New Zealand 57, 295 Pp. Donovan, B. J. 2007
Donovan, B. J. 2007: Apoidea (Insecta: Hymenoptera). Fauna of New Zealand 57, 295 pp. EDITORIAL BOARD REPRESENTATIVES OF L ANDCARE R ESEARCH Dr D. Choquenot Landcare Research Private Bag 92170, Auckland, New Zealand Dr R. J. B. Hoare Landcare Research Private Bag 92170, Auckland, New Zealand REPRESENTATIVE OF UNIVERSITIES Dr R.M. Emberson c/- Bio-Protection and Ecology Division P.O. Box 84, Lincoln University, New Zealand REPRESENTATIVE OF M USEUMS Mr R.L. Palma Natural Environment Department Museum of New Zealand Te Papa Tongarewa P.O. Box 467, Wellington, New Zealand REPRESENTATIVE OF OVERSEAS I NSTITUTIONS Dr M. J. Fletcher Director of the Collections NSW Agricultural Scientific Collections Unit Forest Road, Orange NSW 2800, Australia * * * SERIES EDITOR Dr T. K. Crosby Landcare Research Private Bag 92170, Auckland, New Zealand Fauna of New Zealand Ko te Aitanga Pepeke o Aotearoa Number / Nama 57 Apoidea (Insecta: Hymenoptera) B. J. Donovan Donovan Scientific Insect Research, Canterbury Agriculture and Science Centre, Lincoln, New Zealand [email protected] Manaaki W h e n u a P R E S S Lincoln, Canterbury, New Zealand 2007 4 Donovan (2007): Apoidea (Insecta: Hymenoptera) Copyright © Landcare Research New Zealand Ltd 2007 No part of this work covered by copyright may be reproduced or copied in any form or by any means (graphic, electronic, or mechanical, including photocopying, recording, taping information retrieval systems, or otherwise) without the written permission of the publisher. Cataloguing in publication Donovan, B. J. (Barry James), 1941– Apoidea (Insecta: Hymenoptera) / B. J. Donovan – Lincoln, N.Z. : Manaaki Whenua Press, Landcare Research, 2007. (Fauna of New Zealand, ISSN 0111–5383 ; no. -

UC Berkeley UC Berkeley Electronic Theses and Dissertations
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title Bees and belonging: Pesticide detection for wild bees in California agriculture and sense of belonging for undergraduates in a mentorship program Permalink https://escholarship.org/uc/item/8z91q49s Author Ward, Laura True Publication Date 2020 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California Bees and belonging: Pesticide detection for wild bees in California agriculture and sense of belonging for undergraduates in a mentorship program By Laura T Ward A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Environmental Science, Policy, and Management in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Nicholas J. Mills, Chair Professor Erica Bree Rosenblum Professor Eileen A. Lacey Fall 2020 Bees and belonging: Pesticide detection for wild bees in California agriculture and sense of belonging for undergraduates in a mentorship program © 2020 by Laura T Ward Abstract Bees and belonging: Pesticide detection for wild bees in California agriculture and sense of belonging for undergraduates in a mentorship program By Laura T Ward Doctor of Philosophy in Environmental Science, Policy, and Management University of California, Berkeley Professor Nicholas J. Mills, Chair This dissertation combines two disparate subjects: bees and belonging. The first two chapters explore pesticide exposure for wild bees and honey bees visiting crop and non-crop plants in northern California agriculture. The final chapter utilizes surveys from a mentorship program as a case study to analyze sense of belonging among undergraduates. The first chapter of this dissertation explores pesticide exposure for wild bees and honey bees visiting sunflower crops. -

Global Trends in Bumble Bee Health
EN65CH11_Cameron ARjats.cls December 18, 2019 20:52 Annual Review of Entomology Global Trends in Bumble Bee Health Sydney A. Cameron1,∗ and Ben M. Sadd2 1Department of Entomology, University of Illinois, Urbana, Illinois 61801, USA; email: [email protected] 2School of Biological Sciences, Illinois State University, Normal, Illinois 61790, USA; email: [email protected] Annu. Rev. Entomol. 2020. 65:209–32 Keywords First published as a Review in Advance on Bombus, pollinator, status, decline, conservation, neonicotinoids, pathogens October 14, 2019 The Annual Review of Entomology is online at Abstract ento.annualreviews.org Bumble bees (Bombus) are unusually important pollinators, with approx- https://doi.org/10.1146/annurev-ento-011118- imately 260 wild species native to all biogeographic regions except sub- 111847 Saharan Africa, Australia, and New Zealand. As they are vitally important in Copyright © 2020 by Annual Reviews. natural ecosystems and to agricultural food production globally, the increase Annu. Rev. Entomol. 2020.65:209-232. Downloaded from www.annualreviews.org All rights reserved in reports of declining distribution and abundance over the past decade ∗ Corresponding author has led to an explosion of interest in bumble bee population decline. We Access provided by University of Illinois - Urbana Champaign on 02/11/20. For personal use only. summarize data on the threat status of wild bumble bee species across bio- geographic regions, underscoring regions lacking assessment data. Focusing on data-rich studies, we also synthesize recent research on potential causes of population declines. There is evidence that habitat loss, changing climate, pathogen transmission, invasion of nonnative species, and pesticides, oper- ating individually and in combination, negatively impact bumble bee health, and that effects may depend on species and locality. -

Portland Field Guide
FIELD GUIDE FRITZ HAEG’S ANIMAL ESTATES REGIONAL MODEL HOMES 5.0 PORTLAND, OREGON 5 . N 0 O P G O RE RTLAND, O ANIMAL ESTATES 5.0 PORTLAND, OREGON DOUGLAS F. COOLEY MEMORIAL ART GALLERY REED COLLEGE 26 AUGUST–5 OCTOBER 2008 FRITZ HAEG 04 INTRODUCTION 10 IN LIVABLE CITIES IS FRITZ HAEG PRESERVATION OF THE WILD MIKE HOUCK 06 BUILD A BETTER SNAG! 14 SNAGS AND LOGS STEPHANIE SNYDER CHARLOTTE CORKRAN ANIMAL CLIENTS 18 CLIENT 5.1 34 CLIENT 5.5 VAUX’S SWIFT NORTHWESTERN GARTER SNAKE CHAETURA VAUXI THAMNOPHIS SIRTALIS TETRATAENIA CONTENTS BOB SALLINGER TIERRA CURRY 2 2 CLIENT 5.2 38 CLIENT 5.6 WHITE-BREASTED NUTHATCH ORANGE-RUMPED BUMBLEBEE SITTA CAROLINENSIS BOMBUS MELANOPYGUS CHARLOTTE CORKRAN CHRISTOPHER MARSHALL 26 CLIENT 5.3 42 CLIENT 5.7 OLIVE-SIDED FLYCATCHER SNAIL-EATING GROUND BEETLE CONTOPUS COOPERI SCAPHINOTUS ANGULATUS CHARLOTTE CORKRAN CHRISTOPHER MARSHALL 30 CLIENT 5.4 SILVER-HAIRED BAT LASIONYCTERIS NOCTIVAGANS CHARLOTTE CORKRAN 46 FIELD NOTES 48 CREDITS CLIENT 5.1 VAUX’S SWIFT CHAETURA VAUXI The ongoing Animal Estates lished between the man-made and the wild. initiative creates dwellings for Animals and their habitats are woven back into our cities, strip malls, garages, offi ce parks, animals that have been displaced freeways, backyards, parking lots, and neigh- by humans. Each edition of borhoods. Animal Estates intends to provide a the project is accompanied by provocative twenty-fi rst-century model for the some combination of events, human-animal relationship that is more intimate, visible, and thoughtful. workshops, exhibitions, videos, printed materials, and a temporary PORTLAND headquarters presenting an ever- In the gallery, the temporary Animal Estates expanding urban wildlife archive. -

December 2015 / January 2016
University of Arizona Yavapai County Cooperative Extension Yavapai Gardens Master Gardener Newsletter December 2015 - January 2016 Mushrooms for Your Kitchen and Garden By Lori Dekker The world of mushrooms Events & Activities is entering a new era. In the past gardeners and MG Association Meeting, NO MEETING IN DECEM- foodies have considered BER, next meeting Jan. 20 in Prescott. 6:30pm the mushroom to be a Alta Vista Gardening Club, Prescott, fourth Tues- garden novelty or a tasty day of the month, 12:30pm. Call 928-458-9508 for culinary delight, while a information. few of us have an interest in the possible health benefits or the psy- cho/spiritual/recreational uses of a few of the more famous mush- Prescott Area Gourd Society, third Wednesday of the rooms. For now, I’d like to consider the potential health benefits of month, 10:30am, at Miller Valley Indoor Art Market, fungi in your soil and therefore your garden. 531 Madison Ave, Prescott When left to their own devices mushrooms, or more accu- rately fungi, are decomposers and eventually constructors. In a nut- Prescott Orchid Society, 4rd Sunday of the month, 1pm at the Prescott Library, (928) 717-0623 shell, they build soil from the raw material of litter and waste found in the garden. Since they digest food outside their bodies, they are Prescott Area Iris Society call 928-445-8132 for date essentially “sweating” digestive enzymes and producing waste as and place information. they grow through their environment. To put it more simply and hap- pily for gardeners, the fungus breaks down complex compounds Mountain View Garden Club, Prescott Valley, Dewey into more simple ones that then become available, leaving behind area, 2nd Friday of month, 1:30pm, call 775-4993 for metabolites that can, in turn, be utilized by other microbes. -

References and Appendices
References Ainley, D.G., S.G. Allen, and L.B. Spear. 1995. Off- Arnold, R.A. 1983. Ecological studies on six endan- shore occurrence patterns of marbled murrelets gered butterflies (Lepidoptera: Lycaenidae): in central California. In: C.J. Ralph, G.L. Hunt island biogeography, patch dynamics, and the Jr., M.G. Raphael, and J.F. Piatt, technical edi- design of habitat preserves. University of Cali- tors. Ecology and Conservation of the Marbled fornia Publications in Entomology 99: 1–161. Murrelet. USDA Forest Service, General Techni- Atwood, J.L. 1993. California gnatcatchers and coastal cal Report PSW-152; 361–369. sage scrub: the biological basis for endangered Allen, C.R., R.S. Lutz, S. Demairais. 1995. Red im- species listing. In: J.E. Keeley, editor. Interface ported fire ant impacts on Northern Bobwhite between ecology and land development in Cali- populations. Ecological Applications 5: 632-638. fornia. Southern California Academy of Sciences, Allen, E.B., P.E. Padgett, A. Bytnerowicz, and R.A. Los Angeles; 149–169. Minnich. 1999. Nitrogen deposition effects on Atwood, J.L., P. Bloom, D. Murphy, R. Fisher, T. Scott, coastal sage vegetation of southern California. In T. Smith, R. Wills, P. Zedler. 1996. Principles of A. Bytnerowicz, M.J. Arbaugh, and S. Schilling, reserve design and species conservation for the tech. coords. Proceedings of the international sym- southern Orange County NCCP (Draft of Oc- posium on air pollution and climate change effects tober 21, 1996). Unpublished manuscript. on forest ecosystems, February 5–9, 1996, River- Austin, M. 1903. The Land of Little Rain. University side, CA.