Lecture 2 — Symmetry in the Solid State - Part II: Crystallographic Coordinates and Space Groups
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Screw and Lie Group Theory in Multibody Dynamics Recursive Algorithms and Equations of Motion of Tree-Topology Systems
Multibody Syst Dyn DOI 10.1007/s11044-017-9583-6 Screw and Lie group theory in multibody dynamics Recursive algorithms and equations of motion of tree-topology systems Andreas Müller1 Received: 12 November 2016 / Accepted: 16 June 2017 © The Author(s) 2017. This article is published with open access at Springerlink.com Abstract Screw and Lie group theory allows for user-friendly modeling of multibody sys- tems (MBS), and at the same they give rise to computationally efficient recursive algo- rithms. The inherent frame invariance of such formulations allows to use arbitrary reference frames within the kinematics modeling (rather than obeying modeling conventions such as the Denavit–Hartenberg convention) and to avoid introduction of joint frames. The com- putational efficiency is owed to a representation of twists, accelerations, and wrenches that minimizes the computational effort. This can be directly carried over to dynamics formula- tions. In this paper, recursive O(n) Newton–Euler algorithms are derived for the four most frequently used representations of twists, and their specific features are discussed. These for- mulations are related to the corresponding algorithms that were presented in the literature. Two forms of MBS motion equations are derived in closed form using the Lie group formu- lation: the so-called Euler–Jourdain or “projection” equations, of which Kane’s equations are a special case, and the Lagrange equations. The recursive kinematics formulations are readily extended to higher orders in order to compute derivatives of the motions equations. To this end, recursive formulations for the acceleration and jerk are derived. It is briefly discussed how this can be employed for derivation of the linearized motion equations and their time derivatives. -

Nanocrystalline Nanowires: I. Structure
Nanocrystalline Nanowires: I. Structure Philip B. Allen Department of Physics and Astronomy, State University of New York, Stony Brook, NY 11794-3800∗ and Center for Functional Nanomaterials, Brookhaven National Laboratory, Upton, NY 11973-5000 (Dated: September 7, 2006) Geometric constructions of possible atomic arrangements are suggested for inorganic nanowires. These are fragments of bulk crystals, and can be called \nanocrystalline" nanowires (NCNW). To minimize surface polarity, nearly one-dimensional formula units, oriented along the growth axis, generate NCNW's by translation and rotation. PACS numbers: 61.46.-w, 68.65.La, 68.70.+w Introduction. Periodic one-dimensional (1D) motifs twinned16; \core-shell" (COHN)4,17; and longitudinally are common in nature. The study of single-walled car- heterogeneous (LOHN) NCNW's. bon nanotubes (SWNT)1,2 is maturing rapidly, but other Design principle. The remainder of this note is about 1D systems are at an earlier stage. Inorganic nanowires NCNW's. It offers a possible design principle, which as- are the topic of the present two papers. There is much sists visualization of candidate atomic structures, and optimism in this field3{6. Imaging gives insight into provides a template for arrangements that can be tested self-assembled nanostructures, and incentives to improve theoretically, for example by density functional theory growth protocols. Electron microscopy, diffraction, and (DFT). The basic idea is (1) choose a maximally linear, optical spectroscopy on individual wires7 provide detailed charge-neutral, and (if possible) dipole-free atomic clus- information. Device applications are expected. These ter containing a single formula unit, and (2) using if possi- systems offer great opportunities for atomistic modelling. -

Molecular Symmetry
Molecular Symmetry Symmetry helps us understand molecular structure, some chemical properties, and characteristics of physical properties (spectroscopy) – used with group theory to predict vibrational spectra for the identification of molecular shape, and as a tool for understanding electronic structure and bonding. Symmetrical : implies the species possesses a number of indistinguishable configurations. 1 Group Theory : mathematical treatment of symmetry. symmetry operation – an operation performed on an object which leaves it in a configuration that is indistinguishable from, and superimposable on, the original configuration. symmetry elements – the points, lines, or planes to which a symmetry operation is carried out. Element Operation Symbol Identity Identity E Symmetry plane Reflection in the plane σ Inversion center Inversion of a point x,y,z to -x,-y,-z i Proper axis Rotation by (360/n)° Cn 1. Rotation by (360/n)° Improper axis S 2. Reflection in plane perpendicular to rotation axis n Proper axes of rotation (C n) Rotation with respect to a line (axis of rotation). •Cn is a rotation of (360/n)°. •C2 = 180° rotation, C 3 = 120° rotation, C 4 = 90° rotation, C 5 = 72° rotation, C 6 = 60° rotation… •Each rotation brings you to an indistinguishable state from the original. However, rotation by 90° about the same axis does not give back the identical molecule. XeF 4 is square planar. Therefore H 2O does NOT possess It has four different C 2 axes. a C 4 symmetry axis. A C 4 axis out of the page is called the principle axis because it has the largest n . By convention, the principle axis is in the z-direction 2 3 Reflection through a planes of symmetry (mirror plane) If reflection of all parts of a molecule through a plane produced an indistinguishable configuration, the symmetry element is called a mirror plane or plane of symmetry . -

Chapter 1 – Symmetry of Molecules – P. 1
Chapter 1 – Symmetry of Molecules – p. 1 - 1. Symmetry of Molecules 1.1 Symmetry Elements · Symmetry operation: Operation that transforms a molecule to an equivalent position and orientation, i.e. after the operation every point of the molecule is coincident with an equivalent point. · Symmetry element: Geometrical entity (line, plane or point) which respect to which one or more symmetry operations can be carried out. In molecules there are only four types of symmetry elements or operations: · Mirror planes: reflection with respect to plane; notation: s · Center of inversion: inversion of all atom positions with respect to inversion center, notation i · Proper axis: Rotation by 2p/n with respect to the axis, notation Cn · Improper axis: Rotation by 2p/n with respect to the axis, followed by reflection with respect to plane, perpendicular to axis, notation Sn Formally, this classification can be further simplified by expressing the inversion i as an improper rotation S2 and the reflection s as an improper rotation S1. Thus, the only symmetry elements in molecules are Cn and Sn. Important: Successive execution of two symmetry operation corresponds to another symmetry operation of the molecule. In order to make this statement a general rule, we require one more symmetry operation, the identity E. (1.1: Symmetry elements in CH4, successive execution of symmetry operations) 1.2. Systematic classification by symmetry groups According to their inherent symmetry elements, molecules can be classified systematically in so called symmetry groups. We use the so-called Schönfliess notation to name the groups, Chapter 1 – Symmetry of Molecules – p. 2 - which is the usual notation for molecules. -

Symmetry Indicators and Anomalous Surface States of Topological Crystalline Insulators
PHYSICAL REVIEW X 8, 031070 (2018) Symmetry Indicators and Anomalous Surface States of Topological Crystalline Insulators Eslam Khalaf,1,2 Hoi Chun Po,2 Ashvin Vishwanath,2 and Haruki Watanabe3 1Max Planck Institute for Solid State Research, Heisenbergstraße 1, 70569 Stuttgart, Germany 2Department of Physics, Harvard University, Cambridge, Massachusetts 02138, USA 3Department of Applied Physics, University of Tokyo, Tokyo 113-8656, Japan (Received 15 February 2018; published 14 September 2018) The rich variety of crystalline symmetries in solids leads to a plethora of topological crystalline insulators (TCIs), featuring distinct physical properties, which are conventionally understood in terms of bulk invariants specialized to the symmetries at hand. While isolated examples of TCI have been identified and studied, the same variety demands a unified theoretical framework. In this work, we show how the surfaces of TCIs can be analyzed within a general surface theory with multiple flavors of Dirac fermions, whose mass terms transform in specific ways under crystalline symmetries. We identify global obstructions to achieving a fully gapped surface, which typically lead to gapless domain walls on suitably chosen surface geometries. We perform this analysis for all 32 point groups, and subsequently for all 230 space groups, for spin-orbit-coupled electrons. We recover all previously discussed TCIs in this symmetry class, including those with “hinge” surface states. Finally, we make connections to the bulk band topology as diagnosed through symmetry-based indicators. We show that spin-orbit-coupled band insulators with nontrivial symmetry indicators are always accompanied by surface states that must be gapless somewhere on suitably chosen surfaces. We provide an explicit mapping between symmetry indicators, which can be readily calculated, and the characteristic surface states of the resulting TCIs. -

Symmetry of Graphs. Circles
Symmetry of graphs. Circles Symmetry of graphs. Circles 1 / 10 Today we will be interested in reflection across the x-axis, reflection across the y-axis and reflection across the origin. Reflection across y reflection across x reflection across (0; 0) Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. It is called symmetric if some geometric move preserves it Symmetry of graphs. Circles 2 / 10 Reflection across y reflection across x reflection across (0; 0) Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. It is called symmetric if some geometric move preserves it Today we will be interested in reflection across the x-axis, reflection across the y-axis and reflection across the origin. Symmetry of graphs. Circles 2 / 10 Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. It is called symmetric if some geometric move preserves it Today we will be interested in reflection across the x-axis, reflection across the y-axis and reflection across the origin. Reflection across y reflection across x reflection across (0; 0) Symmetry of graphs. Circles 2 / 10 Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. -

Download English-US Transcript (PDF)
MITOCW | ocw-3.60-13oct2005-pt2-220k_512kb.mp4 PROFESSOR: To mention, most everyone did extremely well on the quiz. But I sense that there's still some of you who have not yet come to terms with crystallographic directions and planes, and you feel a little bit awkward in distinguishing brackets around the HKL and parentheses around HKL. And there are some people who generally get that straightened out, but when I said point group, suddenly pictures of lattices with fourfold axes and twofold axes adorning them came in, and that isn't involved in a point group at also. Again, a point group the symmetry about point. A space group is symmetry spread out through all of space and infinite numbers. So let me say a little bit about resources. I don't know whether you've been following what we've been doing in the notes from Buerger's book that I passed out. That was hard to do is because we did the plane groups, and he doesn't touch them at all. So now we're back following once again Buerger's treatment quite closely. So read the book. And if you like, I can tell you with the end of each lecture, this stuff is on pages 57 through 62. The other thing. As you'll notice, this nonintrusive gentleman in the back is making videotapes of all the lectures. These are eventually going to go up on the website as OpenCourseWare. We were just speaking about that, and it takes a while before they get up, but I have a disk of every lecture. -

Crystallography: Symmetry Groups and Group Representations B
EPJ Web of Conferences 22, 00006 (2012) DOI: 10.1051/epjconf/20122200006 C Owned by the authors, published by EDP Sciences, 2012 Crystallography: Symmetry groups and group representations B. Grenier1 and R. Ballou2 1SPSMS, UMR-E 9001, CEA-INAC / UJF-Grenoble, MDN, 38054 Grenoble, France 2Institut Néel, CNRS / UJF, 25 rue des Martyrs, BP. 166, 38042 Grenoble Cedex 9, France Abstract. This lecture is aimed at giving a sufficient background on crystallography, as a reminder to ease the reading of the forthcoming chapters. It more precisely recalls the crystallographic restrictions on the space isometries, enumerates the point groups and the crystal lattices consistent with these, examines the structure of the space group, which gathers all the spatial invariances of a crystal, and describes a few dual notions. It next attempts to familiarize us with the representation analysis of physical states and excitations of crystals. 1. INTRODUCTION Crystallography covers a wide spectrum of investigations: i- it aspires to get an insight into crystallization phenomena and develops methods of crystal growths, which generally pertains to the physics of non linear irreversible processes; ii- it geometrically describes the natural shapes and the internal structures of the crystals, which is carried out most conveniently by borrowing mathematical tools from group theory; iii- it investigates the crystallized matter at the atomic scale by means of diffraction techniques using X-rays, electrons or neutrons, which are interpreted in the dual context of the reciprocal space and transposition therein of the crystal symmetries; iv- it analyzes the imperfections of the crystals, often directly visualized in scanning electron, tunneling or force microscopies, which in some instances find a meaning by handling unfamiliar concepts from homotopy theory; v- it aims at providing means for discerning the influences of the crystal structure on the physical properties of the materials, which requires to make use of mathematical methods from representation theory. -
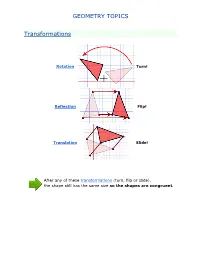
Geometry Topics
GEOMETRY TOPICS Transformations Rotation Turn! Reflection Flip! Translation Slide! After any of these transformations (turn, flip or slide), the shape still has the same size so the shapes are congruent. Rotations Rotation means turning around a center. The distance from the center to any point on the shape stays the same. The rotation has the same size as the original shape. Here a triangle is rotated around the point marked with a "+" Translations In Geometry, "Translation" simply means Moving. The translation has the same size of the original shape. To Translate a shape: Every point of the shape must move: the same distance in the same direction. Reflections A reflection is a flip over a line. In a Reflection every point is the same distance from a central line. The reflection has the same size as the original image. The central line is called the Mirror Line ... Mirror Lines can be in any direction. Reflection Symmetry Reflection Symmetry (sometimes called Line Symmetry or Mirror Symmetry) is easy to see, because one half is the reflection of the other half. Here my dog "Flame" has her face made perfectly symmetrical with a bit of photo magic. The white line down the center is the Line of Symmetry (also called the "Mirror Line") The reflection in this lake also has symmetry, but in this case: -The Line of Symmetry runs left-to-right (horizontally) -It is not perfect symmetry, because of the lake surface. Line of Symmetry The Line of Symmetry (also called the Mirror Line) can be in any direction. But there are four common directions, and they are named for the line they make on the standard XY graph. -

Symmetry in Chemistry - Group Theory
Symmetry in Chemistry - Group Theory Group Theory is one of the most powerful mathematical tools used in Quantum Chemistry and Spectroscopy. It allows the user to predict, interpret, rationalize, and often simplify complex theory and data. At its heart is the fact that the Set of Operations associated with the Symmetry Elements of a molecule constitute a mathematical set called a Group. This allows the application of the mathematical theorems associated with such groups to the Symmetry Operations. All Symmetry Operations associated with isolated molecules can be characterized as Rotations: k (a) Proper Rotations: Cn ; k = 1,......, n k When k = n, Cn = E, the Identity Operation n indicates a rotation of 360/n where n = 1,.... k (b) Improper Rotations: Sn , k = 1,....., n k When k = 1, n = 1 Sn = , Reflection Operation k When k = 1, n = 2 Sn = i , Inversion Operation In general practice we distinguish Five types of operation: (i) E, Identity Operation k (ii) Cn , Proper Rotation about an axis (iii) , Reflection through a plane (iv) i, Inversion through a center k (v) Sn , Rotation about an an axis followed by reflection through a plane perpendicular to that axis. Each of these Symmetry Operations is associated with a Symmetry Element which is a point, a line, or a plane about which the operation is performed such that the molecule's orientation and position before and after the operation are indistinguishable. The Symmetry Elements associated with a molecule are: (i) A Proper Axis of Rotation: Cn where n = 1,.... This implies n-fold rotational symmetry about the axis. -

Chapter 1 Rigid Body Kinematics
Chapter 1 Rigid Body Kinematics 1.1 Introduction This chapter builds up the basic language and tools to describe the motion of a rigid body – this is called rigid body kinematics. This material will be the foundation for describing general mech- anisms consisting of interconnected rigid bodies in various topologies that are the focus of this book. Only the geometric property of the body and its evolution in time will be studied; the re- sponse of the body to externally applied forces is covered in Chapter ?? under the topic of rigid body dynamics. Rigid body kinematics is not only useful in the analysis of robotic mechanisms, but also has many other applications, such as in the analysis of planetary motion, ground, air, and water vehicles, limbs and bodies of humans and animals, and computer graphics and virtual reality. Indeed, in 1765, motivated by the precession of the equinoxes, Leonhard Euler decomposed a rigid body motion to a translation and rotation, parameterized rotation by three principal-axis rotation (Euler angles), and proved the famous Euler’s rotation theorem [?]. A body is rigid if the distance between any two points fixed with respect to the body remains constant. If the body is free to move and rotate in space, it has 6 degree-of-freedom (DOF), 3 trans- lational and 3 rotational, if we consider both translation and rotation. If the body is constrained in a plane, then it is 3-DOF, 2 translational and 1 rotational. We will adopt a coordinate-free ap- proach as in [?, ?] and use geometric, rather than purely algebraic, arguments as much as possible. -

Script Unit 4.4 (Screw Axes in Crystal Structures).Docx
Script Unit 4.4 Welcome back! Slide 2 In the last unit, we introduced helical or screw symmetry in general; now, we want to investigate, what different kinds of screw axes, that is, what kind of different helical symmetries are present in crystal structures. Slide 3 Let’s look at a first example - here you see a unit cell of a crystal in two different views, a crystal, which should belong to the hexagonal crystal system; the right view is a projection along the c- direction. We see, that this crystal is composed of atoms, that build these helices running along all corners of the unit cell; if we look from above onto this plane, then these screws look like flat hexagons. Because this is a crystal we have translational symmetry, these helices are repeated again and again as a whole, here along the a- and b-direction. But there is - likewise as in glide planes - another symmetry element present, which has a translational component being smaller than a whole unit cell - and this is a screw axis. Slide 4 Along this axis the atoms are symmetry related to each other by applying a screw rotation: if we rotate first this atom by 60 degrees and then translate it parallel to the screw axis by one-sixth of the unit cell, then it will be mapped onto this atom. This can be done with all other atoms as well with this one, that one and so forth… This is an example of a six-fold screw axis, meaning the rotational part is 60 degrees - analogous to pure rotations.