A Review of Selected Federal Vaccine and Immunization Policies, Based on Case Studies of Pneumococcal Vaccine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

0 January to July 2021
0 www.journalsofindia.com January to July 2021 SCIENCE & TECH ............................................................................................................................................................... 6 1. REUSABLE LAUNCH VEHICLE TECHNOLOGY DEMONSTRATION PROGRAMME(RLV-TD) ................................................. 6 2. GAGANYAAN MISSION ..................................................................................................................................................... 6 3. MARS ORBITER MISSION (MOM) ..................................................................................................................................... 6 4. CHANDRAYAAN MISSION................................................................................................................................................. 7 5. SOLAR MISSION ............................................................................................................................................................... 8 6. ARTEMIS ACCORD ............................................................................................................................................................ 9 7. NATIONAL MISSION ON INTERDISCIPLINARY CYBER-PHYSICAL SYSTEM (NMICPS) ....................................................... 10 8. SMART ANTI-AIRFIELD WEAPON (SAAW) ...................................................................................................................... 10 9. AQUAPONICS ................................................................................................................................................................ -

View Introduction and Presentation from Dr
1 We are here tonight to honor and celebrate the outstanding accomplishments and contributions in the field of vaccines of our colleague, our mentor, our friend, Mathu Santosham. Mathu’s professional titles attest to his accomplishments at the highest levels. He is professor of Pediatrics and International Health at Johns Hopkins University and is the Founder and Director of the Center for American Indian Health. 2 He is a widely recognized and celebrated international expert in the area of vaccines against rotavirus, pneumococcus and Haemophilus influenzae type b----the area of work and contribution for which he is being awarded this year’s Sabin Gold Medal Award. But he has also made equally impactful contributions in the area of oral rehydration therapy for diarrheal disease, neonatal survival strategies and more broadly in the area of reducing health disparities for this country’s first nations American Indian people. Millions of deaths around the world have been prevented because of his medical and scientific contributions. Ostensibly this is what we are here to celebrate. 3 Mathu has been celebrated with numerous awards from the Indian Health Service, the Thrasher Research Fund, and the pneumococcal scientific community through the Robert Austrian Award along with awards from his own institution to recognize him from among the many outstanding alumnae of the school. 4 His work and sage advice valued by many around the world, including shown here ABC’s Chief Health and Medical Editor, Dr. Rich Besser. 5 Here with Martin Sheen at the Native Vision Camp in 2012. 6 Here working with Robert Redford on American Indian health disparity issues. -

Journal of Vaccines & Vaccination
ccines & a V f V a o c l c i a n n a r t u i o o n J Ching, et al., J Vaccines Vaccin 2014, 5:6 Journal of Vaccines & Vaccination DOI: 10.4172/2157-7560.1000257 ISSN: 2157-7560 Research Article Open Access Evaluation of a Recombinant Vaccine Candidate r56Lc-1 in a Chigger Challenge Mouse Model Wei-Mei Ching1,3*, Woradee Lurchachaiwong2, Zhiwen Zhang1,3 Temitayo Awoyomi1,3, Chien-Chung Chao1,3 and Anthony Schuster2 1Viral and Rickettsial Diseases Department, Infectious Diseases Directorate, Naval Medical Research Center, Silver Spring, USA 2Entomology Department, Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand 3Uniformed Services University of the Health Sciences, Bethesda, USA *Corresponding author: Wei-Mei Ching, PhD, Viral and Rickettsial Diseases Department, Infectious Diseases Directorate, Naval Medical Research Center, 503 Robert Grant Ave, RM3N71, Silver Spring, MD 20910, USA, Tel: 301 319 7438; Fax: 301 319 7451; E-mail: [email protected] Received date: 15 Sep 2014; Accepted date: 24 Oct 2014; Published date: 27 Oct 2014 Copyright: © 2014 Ching WM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Scrub typhus, an acute, febrile disease is transmitted by the bite of an Orientia infected chigger. We evaluated the protective potential of a recombinant 56 kDa antigen in a chigger challenge mouse model which mimics the natural transmission of Orientia. Chiggers from an L. -

Vaccination and Anaphylaxis
14 FORENSIC SCIENCE Croat Med J. 2017;58:14-25 https://doi.org/10.3325/cmj.2017.58.14 Vaccination and anaphylaxis: a Cristian Palmiere1, Camilla Tettamanti2, Maria Pia forensic perspective Scarpelli1 1CURML, University Center of Legal Medicine, Lausanne 25, Switzerland 2Department of Legal Medicine, Aim To review the available literature pertaining to fatali- University of Genova, Genova, Italy ties following vaccine administration and, in particular, cas- es of vaccine-related fatal anaphylaxis. Method The MEDLINE database was systematically searched up to March 2016 to identify all relevant articles pertaining to fatal cases of anaphylaxis following vaccine administration. Results Six papers pertaining to fatal anaphylaxis following vaccination were found relevant. Mast cell tryptase and to- tal IgE concentration was assessed exclusively in one case. Laryngeal edema was not detected in any of these cases, whereas eosinophil or mast cell infiltration was observed in lymphoid organs. In one case, immunohistochemical in- vestigations using anti-tryptase antibodies allowed pulmo- nary mast cells and degranulating mast cells with tryptase- positive material outside to be identified. Conclusion In any suspected IgE-mediated fatal anaphy- lactic cases, biochemical investigations should be system- atically performed for forensic purposes. Splenic tissue should be routinely sampled for immunohistochemical investigations in all suspected anaphylaxis-related deaths and mast cell/eosinophil infiltrations should be systemati- cally sought out in -

India: the World's Pharmacy Expands Its Reach in Global Health
New York | New Delhi | Rio de Janeiro Nairobi | Johannesburg | London India: The World’s Pharmacy Expands Its Reach in Global Health March 2021 This white paper was last updated on 1 March 2021 Global Health Strategies 18/1, 2nd Floor Shaheed Bhawan, Aruna Asaf Ali Marg, New Delhi, 110 067 www.globalhealthstrategies.com Twitter: @GHS Contents Executive Summary 02 India’s response to COVID-19 03 ∙ Pharmaceuticals and Biosimilars 04 ∙ Vaccines 05 ∙ Diagnostics 06 Evolution of India’s pharmaceutical industry 07 ∙ Milestones 08 Case Studies ∙ Hepatitis B Vaccine 10 ∙ Anti-retroviral Drugs 11 ∙ MenAfriVac 12 ∙ Complex Generics 13 ∙ Insulin 14 ∙ Monoclonal Antibodies 15 ∙ Vaccine for Rotavirus 16 ∙ Typhoid Conjugate Vaccine 17 Conclusion: Looking Forward 18 Executive Summary India’s pharmaceutical industry is already playing a pivotal role in the scale-up of pharmaceuticals and diagnostics to combat the global COVID-19 pandemic. It is poised to play an even more dominant role as biological products – preventive vaccines and cutting-edge biotechnology such as monoclonal antibodies– come to the fore. Even before the pandemic, Indian manufacturers produced vast quantities of generic antiviral drugs that turned HIV from a death sentence to a chronic manageable condition in developing countries. India’s global dominance in generic drugs and vaccine manufacturing has earned it the label “Pharmacy of the World”. COVID-19 only strengthens the case for this moniker. So far, India has supplied medicines to 133 countries to fight the pandemic. Six Indian manufacturers have been granted royalty-free licenses by Gilead to manufacture the first antiviral drug approved by the U.S. -

Contributions of Native Americans to the Global Control of Infectious Diseases Mathuram Santosham A,∗, Raymond Reid A, Aruna Chandran A, Eugene V
Vaccine 25 (2007) 2366–2374 Contributions of Native Americans to the global control of infectious diseases Mathuram Santosham a,∗, Raymond Reid a, Aruna Chandran a, Eugene V. Millar a, James P. Watt a, Robert Weatherholtz a, Connie Donaldson a, Janne´ Croll a, Lawrence H. Moulton a, Claudette M. Thompson b, George R. Siber c, Katherine L. O’Brien a a Center for American Indian Health, Department of International Health, Johns Hopkins University, 621 N. Washington Street, Baltimore, MD 21205, United States b Department of Epidemiology, Harvard School of Public Health, 665 Huntington Avenue, Boston, MA 02115, United States c Wyeth Vaccines, 401 North Middletown Road, BH 21101, Pearl River, NY 10965, United States Available online 18 September 2006 Abstract For over a half of a century, Native American populations have participated in numerous studies regarding the epidemiology, prevention and treatment of infectious diseases. These studies have resulted in measures to prevent morbidity and mortality from many infectious diseases. The lessons learned from these studies and their resultant prevention or treatment interventions have been applied around the world, and have had a major impact in the reduction of global childhood morbidity and mortality. © 2006 Elsevier Ltd. All rights reserved. Keywords: Native Americans; Infectious diseases; Clinical trials; Pneumococcus; H. influenzae type b (Hib); Rotavirus; Trachoma; Tuberculosis; BCG Key messages from these studies has been applied in many populations around the world and has met with great success. This In the keynote address (The Robert Austrian Lecture) paper reviews the major achievements in Native Amer- for the 5th International Symposium on Pneumococci and ican health that have been made to date. -

Production Flow Sheet
(Refer SOP No. QA-INS-010) Central Drugs Standard Control Organization Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India FDA Bhavan, ITO, Kotla Road, New Delhi -110002 Central Inspection Plan Using Risk Based Approach Of Vaccine Manufacturing Units for 2019 Sl. Name and Vaccines manufactured (category Dt. of last Purpose of last Major Compliance AEFI reported/ Proposed time of No. address of the wise list) inspection inspection (grant deficiencies met till date, changes/ Inspection manufacturing with no of /renewal /post-approval detected if any Product site days & team changes, AEFI, follow- complaints, up, routine) failure if any A NORTH ZONE 1 M/s Biomed Pvt. 1.Oral Polio Vaccine IP, 03.10.2018 to To find out the root As per report Observations NSQ Reported 1st Quarter Ltd, 2. Vi olysaccharide Typhoid Vaccine 06.10.2018 & cause for the NSQ communicate Annual Inspection C-96, Site –I, (Bio TyphTM) IP, 3. Haemophilus Type- 08.10.2018 Reported d to the firm Bulandshahar B Conjugate Vaccine (PedaHib) for 2nd Quarter 4. Meningococal Polysaccharide Vaccine ADC(I) and compliance. Follow-up Road, Ind. Area, (Group A,C,Y,W 135) QuadriMeningo Ghaziabad. 5. Meningococal Polysaccharide Vaccine DIs of Inspection (Group A&C) Bivalent CDSCO, , and (if any) 6. Vi Conjugate Typhoid Vaccine (Peda DI, Uttar Typh), Pradesh 3rd Quarter 7. Vi Conjugate Typhoid Vaccine (Bio Annual Inspection TyphTM), 8. Haemophilus Type-B Conjugate 4th Quarter Vaccine (PedaHib) IP Follow-up 9. Meningococal Inspection Polysaccharide Vaccine (Group A,C,Y,W 135) IP QuadriMeningo (if any) 10. -

Requirements for Diphtheria. Tetanus. Pertussis and Combined Vaccines
0World Health Organization World Health Or_panizatior?. Technics; Rsporr Series. Xo . S0.l . 1990 Annex 2 REQUIREMENTS FOR DIPHTHERIA. TETANUS. PERTUSSIS AND COMBINED VACCINES (Requirements for Biological Substances Nos. 8 and 10) (Revised 1989) Page Introduction ....................................................................................................... 88 Requirements for diphtheria vaccine (adsorbed) ............................................ 91 General considerations ........................................................................... 91 Part A . Manufacturing requirements .......................................................... A .1 Definit~ons............................................................................. A.2 General manufacturing requirements .................................... A.3 Production control ................................................................ A.4 Filling and containers ........................................................... A.5 Control of final product ........................................................ A.6 Records ................................................................................. A.7 Samples ................................................................................. A.8 Labelling ................................................................................ A.9 Distribution and transport .................................................... A.10 Stability . storage and expir>-date ..................................... Part B . Kational control -

WHO Expert Committee on Biological Standardization of Publications
WHOWHOWHO Technical Technical Technical Report Report Report Series Series Series 941941941 BIOLOGICAL BIOLOGICAL BIOLOGICAL ThisThisThis report report report presents presents presents the the recommendationsthe recommendations recommendations of ofa ofWHOa WHOa WHO Expert Expert Expert WHOWHOWHO EXPERT EXPERT EXPERT COMMITTEE COMMITTEE COMMITTEE CommitteeCommitteeCommittee commissioned commissioned commissioned to tocoordinate tocoordinate coordinate activities activities activities leading leading leading to tothe tothe adoptionthe adoption adoption of ofinternational ofinternational international recommendations recommendations recommendations for for the for the the ONONON BIOLOGICAL BIOLOGICAL BIOLOGICAL productionproductionproduction and and andcontrol control control of ofvaccines ofvaccines vaccines and and andother other other biologicals biologicals biologicals STANDARDIZATION STANDARDIZATION STANDARDIZATION andand andthe the establishmentthe establishment establishment of ofinternational ofinternational international biological biological biological reference reference reference STANDARDIZATIONSTANDARDIZATIONSTANDARDIZATION materials.materials.materials. TheThe Thereport report report starts starts starts with with with a discussiona discussiona discussion of ofgeneral ofgeneral general issues issues issues brought brought brought to tothe tothe attentionthe attention attention of ofthe ofthe Committeethe Committee Committee and and andprovides provides provides information information information onon theon the statusthe -

The Historical Impact of Epidemic Typhus
THE HISTORICAL IMPACT OF EPIDEMIC TYPHUS by JOSEPH M. CONLON LCDR, MSC, USN [email protected] INTRODUCTION Louse-borne Typhus Fever is undoubtedly one of the oldest pestilential diseases of mankind. Called by many names and confused with other fevers, it is not until the late fifteenth century that it can be recognized with certainty as causing devastating epidemics. With Plague, Typhoid, and Dysentery, it was the scourge of armies and civilian populations throughout the Middle Ages and frequently played a decisive role in wars conducted in Europe from the 15th through the 20th centuries. The manner in which the course of European history has been affected by Typhus epidemics has been graphically portrayed by a number of authors. This paper will attempt a further analysis of the historical impact of Louse-borne Typhus and how its epidemic propagation has led many to regard Pediculus humanus corporis as having a more profound effect on human history than any other animal. EPIDEMIC TYPHUS FEVER (TABARILLO, CLASSIC OR EUROPEAN TYPHUS, JAIL FEVER, WAR FEVER) Causative Agent. Typhus fever is an acute specific infection caused by Rickettsia prowazeki as isolated and identified by DaRocha-Lima in 1916. Named in honor of H. T. Ricketts and L. von Prowazek, both of whom contracted typhus in the course of their investigations and died, R. prowazeki was originally believed to be a virus because of its minute size and difficulty of cultivation. R. prowazeki is now recognized as being morphologically and biochemically a type of bacterium. A rod-shaped microorganism, R. prowazeki is an obligate intracellular parasite whose cell wall contains muramic acid, diaminopimelic acid, and other components similar to those of the gram-negative bacteria. -
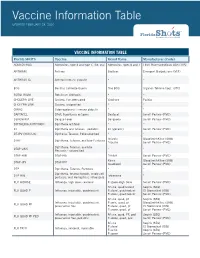
Vaccine Information Table UPDATED FEBRUARY 28, 2020
Vaccine Information Table UPDATED FEBRUARY 28, 2020 VACCINE INFORMATION TABLE Florida SHOTS Vaccine Brand Name Manufacturer (Code) ADENOVIRUS Adenovirus, type 4 and type 7, live, oral Adenovirus Types 4 and 7 TEVA Pharmaceuticals USA (TVA) ANTHRAX Anthrax Biothrax Emergent BioSolutions (MIP) ANTHRAX IG Anthrax immune globulin * * BCG Bacillus Calmette-Guerin Tice BCG Organon Teknika Corp. (OTC) BOTULINUM Botulinum Antitoxin * * CHOLERA LIVE Cholera, live attenuated Vaxchora PaxVax CHOLERA UNK Cholera, unspecified * * CMVIG Cytomegalovirus immune globulin * * DAPTACEL DTaP, 5 pertussis antigens Daptacel Sanofi Pasteur (PMC) DENGVAXIA Dengue Fever Dengvaxia Sanofi Pasteur (PMC) DIPTHERIA ANTITOXIN Diphtheria antitoxin * * DT Diphtheria and Tetanus - pediatric DT (generic) Sanofi Pasteur (PMC) DT-IPV (NON-US) Diptheria, Tetanus, Polio adsorbed * * Infanrix GlaxoSmithKline (SKB) DTAP Diphtheria, Tetanus, acellular Pertussis Tripedia Sanofi Pasteur (PMC) Diphtheria, Tetanus, acellular DTAP UNK * * Pertussis - unspecified DTAP-HIB DTaP-Hib Trihibit Sanofi Pasteur (PMC) Kinrix GlaxoSmithKline (SKB) DTAP-IPV DTaP-IPV Quadracel Sanofi Pasteur (PMC) DTP Diphtheria, Tetanus, Pertussis * * Diphtheria, tetanux toxoids, whole cell DTP-HIB Tetramune * pertussis, and Hemophilus influenza B FLU HIDOSE Influenza, high dose, seasonal Fluzone-High Dose Sanofi Pasteur (PMC) Afluria, quadrivalent Seqiris (SEQ) FLU QUAD P Influenza, injectable, quadrivalent Flulaval, quadrivalent ID Biomedical (IDB) Fluzone, quadrivalent Sanofi Pasteur (PMC) Afluria, quad, pf -

A Century of Pneumococcal Vaccination Research in Humans
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector REVIEW 10.1111/j.1469-0691.2012.03943.x A century of pneumococcal vaccination research in humans J. D. Grabenstein1 and K. P. Klugman2,3 1) Merck Vaccines, West Point, PA USA, 2) Rollins School of Public Health and Division of Infectious Diseases, School of Medicine, Emory University, Atlanta, GA, USA and 3) Medical Research Council, National Institute for Communicable Diseases Respiratory and Meningeal Pathogens Research Unit, University of the Witwatersrand, Johannesburg, South Africa Abstract Sir Almroth Wright coordinated the first trial of a whole-cell pneumococcal vaccine in South Africa from 1911 to 1912. Wright started a chain of events that delivered pneumococcal vaccines of increasing clinical and public-health value, as medicine advanced from a vague understanding of the germ theory of disease to today’s rational vaccine design. Early whole-cell pneumococcal vaccines mimicked early typhoid vaccines, as early pneumococcal antisera mimicked the first diphtheria antitoxins. Pneumococcal typing systems developed by Franz Neufeld and others led to serotype-specific whole-cell vaccines. Pivotally, Alphonse Dochez and Oswald Avery isolated pneumo- coccal capsular polysaccharides in 1916–17. Serial refinements permitted Colin MacLeod and Michael Heidelberger to conduct a 1944– 45 clinical trial of quadrivalent pneumococcal polysaccharide vaccine (PPV), demonstrating a high degree of efficacy in soldiers against pneumococcal pneumonia. Two hexavalent PPVs were licensed in 1947, but were little used as clinicians preferred therapy with new antibiotics, rather than pneumococcal disease prevention. Robert Austrian’s recognition of high pneumococcal case-fatality rates, even with antibiotic therapy, led to additional trials in South Africa, the USA and Papua New Guinea, with 14-valent and 23-valent PPVs licensed in 1977 and 1983 for adults and older children.