Headspace Raman Spectroscopy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vibrational-Rotational Spectroscopy ( )
Vibrational-Rotational Spectroscopy Vibrational-Rotational Spectrum of Heteronuclear Diatomic Absorption of mid-infrared light (~300-4000 cm-1): • Molecules can change vibrational and rotational states • Typically at room temperature, only ground vibrational state populated but several rotational levels may be populated. • Treating as harmonic oscillator and rigid rotor: subject to selection rules ∆v = ±1 and ∆J = ±1 EEEfield=∆ vib +∆ rot =ω =−EEfi = EvJEvJ()′,, ′ −( ′′ ′′ ) ω 11 vvvBJJvvBJJ==00()′′′′′′′′′ +++−++()11() () + 2πc 22 At room temperature, typically v=0′′ and ∆v = +1: ′ ′ ′′ ′′ vv=+0 BJJ() +−11 JJ( +) Now, since higher lying rotational levels can be populated, we can have: ∆=+J 1 JJ′′′=+1 vv= ++21 BJ( ′′ ) R − branch 0 P− branch ∆=−J 1 JJ′′′=−1 vv=−0 2 BJ′′ J’=4 J’=3 J’=1 v’=1 J’=0 P branch Q branch J’’=3 12B J’’=2 6B J’’=1 2B v’’=0 J’’=0 EJ” =0 2B 2B 4B 2B 2B 2B -6B -4B -2B +2B +4B +8B ν ν 0 By measuring absorption splittings, we can get B . From that, the bond length! In polyatomics, we can also have a Q branch, where ∆J0= and all transitions lie at ν=ν0 . This transition is allowed for perpendicular bands: ∂µ ∂q ⊥ to molecular symmetry axis. Intensity of Vibrational-Rotational Transitions There is generally no thermal population in upper (final) state (v’,J’) so intensity should scale as population of lower J state (J”). ∆=NNvJNvJ(,′ ′′′′′′′ ) − (, ) ≈ NJ ( ) NJ()′′∝− gJ ()exp( ′′ EJ′′ / kT ) =+()21exp(J′′ −hcBJ ′′() J ′′ + 1/) kT 5.33 Lecture Notes: Vibrational-Rotational Spectroscopy Page 2 Rotational Populations at Room Temperature for B = 5 cm-1 gJ'' thermal population NJ'' 0 5 10 15 20 Rotational Quantum Number J'' So, the vibrational-rotational spectrum should look like equally spaced lines about ν 0 with sidebands peaked at J’’>0. -
Optical Spectroscopy - Processes Monitored UV/ Fluorescence/ IR/ Raman/ Circular Dichroism
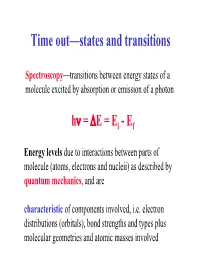
Time out—states and transitions Spectroscopy—transitions between energy states of a molecule excited by absorption or emission of a photon hν = ∆E = Ei -Ef Energy levels due to interactions between parts of molecule (atoms, electrons and nucleii) as described by quantum mechanics, and are characteristic of components involved, i.e. electron distributions (orbitals), bond strengths and types plus molecular geometries and atomic masses involved Spectroscopic Regions Typical wavelength Approximate energy Spectroscopic region Techniques and Applications (cm) (kcal mole-1) -11 8 10 3 x 10 γ-ray MÖssbauer 10-8 3 x 105 X-ray x-ray diffraction, scattering 10-5 3 x 102 Vacuum UV Electronic Spectra 3 x 10-5 102 Near UV Electronic Spectra 6 x 10-5 5 x 103 Visible Electronic Spectra 10-3 3 x 100 IR Vibrational Spectra 10-2 3 x 10-1 Far IR Vibrational Spectra 10-1 3 x 10-2 Microwave Rotational Spectra 100 3 x 10-3 Microwave Electron paramagnetic resonance 10 3 x 10-4 Radio frequency Nuclear magnetic resonance Adapted from Table 7-1; Biophysical Chemistry, Part II by Cantor and Schimmel Spectroscopic Process • Molecules contain distribution of charges (electrons and nuclei, charges from protons) and spins which is dynamically changed when molecule is exposed to light •In a spectroscopic experiment, light is used to probe a sample. What we seek to understand is: – the RATE at which the molecule responds to this perturbation (this is the response or spectral intensity) – why only certain wavelengths cause changes (this is the spectrum, the wavelength dependence of the response) – the process by which the molecule alters the radiation that emerges from the sample (absorption, scattering, fluorescence, photochemistry, etc.) so we can detect it These tell us about molecular identity, structure, mechanisms and analytical concentrations Magnetic Resonance—different course • Long wavelength radiowaves are of low energy that is sufficient to ‘flip’ the spin of nuclei in a magnetic field (NMR). -

A Comparison of Ultraviolet and Visible Raman Spectra of Supported Metal Oxide Catalysts
8600 J. Phys. Chem. B 2001, 105, 8600-8606 A Comparison of Ultraviolet and Visible Raman Spectra of Supported Metal Oxide Catalysts Yek Tann Chua,† Peter C. Stair,*,† and Israel E. Wachs*,‡ Department of Chemistry, Center for Catalysis and Surface Science and Institute of EnVironmental Catalysis, Northwestern UniVersity, EVanston, Illinois 60208, and Zettlemoyer Center for Surface Studies and Department of Chemical Engineering, Lehigh UniVersity, Bethlehem, PennsylVania 18015 ReceiVed: April 11, 2001 The recent emergence of ultraviolet-wavelength-excited Raman spectroscopy as a tool for catalyst characterization has motivated the question of how UV Raman spectra compare to visible-wavelength-excited Raman spectra on the same catalyst system. Measurements of Raman spectra from five supported metal oxide systems (Al2O3-supported Cr2O3,V2O5, and MoO3 as well as TiO2-supported MoO3 and Re2O7), using visible (514.5 nm) and ultraviolet (244 nm) wavelength excitation have been compared to determine the similarities and differences in Raman spectra produced at the two wavelengths. The samples were in the form of self-supporting disks. Spectra from the oxides, both hydrated as a result of contact with ambient air and dehydrated as a result of calcination or laser-induced heating, were recorded. A combination of sample spinning and translation to produce a spiral pattern of laser beam exposure to the catalyst disk was found to be most effective in minimizing dehydration caused by laser-induced heating. Strong absorption by the samples in the ultraviolet significantly reduced the number of scatterers contributing to the Raman spectrum while producing only modest increases in the Raman scattering cross section due to resonance enhancement. -

Rotational Spectroscopy and Interstellar Molecules
Volume -5, Issue-2, April 2015 » Plescia, J. B., and Cintala M. J. (2012), Impact melt Rotational Spectroscopy and Interstellar in small lunar highland craters, J. Geophys. Res., 117, Molecules E00H12,doi:10.1029/2011JE003941 » Plescia J.B. and Spudis P.D. (2014) Impact melt flows at The fact that life exists on Earth is no secret. However, Lowell crater, Planetary and Space Science, 103, 219- understanding the origin of life, its evolution, and the fu- 227 ture of life on Earth remain interesting issues to be ad- » Shkuratov, Y.,Kaydash, V.,Videen, G. (2012)The lunar dressed. That the regions between stars contain by far the crater Giordano Bruno as seen with optical roughness largest reservoir of chemically-bonded matter in nature imagery, Icarus, 218(1), 525-533 obviously demonstrates the importance of chemistry in » Smrekar S. and Pieters C.M. (1985) Near-infrared spec- the interstellar space. The unique detection of over 200 troscopy of probable impact melt from three large lunar different interstellar molecules largely via their rotational highland craters, Icarus, 63, 442-452 spectra has laid to rest the popular perception that the » Srivastava, N., Kumar, D., Gupta, R. P., 2013. Young vastness of space is an empty vacuum dotted with stars, viscous flows in the Lowell crater of Orientale basin, planets, black holes, and other celestial formations. As- Moon: Impact melts or volcanic eruptions? Planetary trochemistry comprises observations, theory and experi- and Space Science, 87, 37-45. ments aimed at understanding the formation of molecules » Stopar J. D., Hawke B. R., Robinson M. S., DeneviB.W., and matter in the Universe i.e. -

Atomic and Molecular Laser-Induced Breakdown Spectroscopy of Selected Pharmaceuticals
Article Atomic and Molecular Laser-Induced Breakdown Spectroscopy of Selected Pharmaceuticals Pravin Kumar Tiwari 1,2, Nilesh Kumar Rai 3, Rohit Kumar 3, Christian G. Parigger 4 and Awadhesh Kumar Rai 2,* 1 Institute for Plasma Research, Gandhinagar, Gujarat-382428, India 2 Laser Spectroscopy Research Laboratory, Department of Physics, University of Allahabad, Prayagraj-211002, India 3 CMP Degree College, Department of Physics, University of Allahabad, Pragyagraj-211002, India 4 Physics and Astronomy Department, University of Tennessee, University of Tennessee Space Institute, Center for Laser Applications, 411 B.H. Goethert Parkway, Tullahoma, TN 37388-9700, USA * Correspondence: [email protected]; Tel.: +91-532-2460993 Received: 10 June 2019; Accepted: 10 July 2019; Published: 19 July 2019 Abstract: Laser-induced breakdown spectroscopy (LIBS) of pharmaceutical drugs that contain paracetamol was investigated in air and argon atmospheres. The characteristic neutral and ionic spectral lines of various elements and molecular signatures of CN violet and C2 Swan band systems were observed. The relative hardness of all drug samples was measured as well. Principal component analysis, a multivariate method, was applied in the data analysis for demarcation purposes of the drug samples. The CN violet and C2 Swan spectral radiances were investigated for evaluation of a possible correlation of the chemical and molecular structures of the pharmaceuticals. Complementary Raman and Fourier-transform-infrared spectroscopies were used to record the molecular spectra of the drug samples. The application of the above techniques for drug screening are important for the identification and mitigation of drugs that contain additives that may cause adverse side-effects. Keywords: paracetamol; laser-induced breakdown spectroscopy; cyanide; carbon swan bands; principal component analysis; Raman spectroscopy; Fourier-transform-infrared spectroscopy 1. -

Rotational Spectroscopy
Applied Spectroscopy Rotational Spectroscopy Recommended Reading: 1. Banwell and McCash: Chapter 2 2. Atkins: Chapter 16, sections 4 - 8 Aims In this section you will be introduced to 1) Rotational Energy Levels (term values) for diatomic molecules and linear polyatomic molecules 2) The rigid rotor approximation 3) The effects of centrifugal distortion on the energy levels 4) The Principle Moments of Inertia of a molecule. 5) Definitions of symmetric , spherical and asymmetric top molecules. 6) Experimental methods for measuring the pure rotational spectrum of a molecule Microwave Spectroscopy - Rotation of Molecules Microwave Spectroscopy is concerned with transitions between rotational energy levels in molecules. Definition d Electric Dipole: p = q.d +q -q p H Most heteronuclear molecules possess Cl a permanent dipole moment -q +q e.g HCl, NO, CO, H2O... p Molecules can interact with electromagnetic radiation, absorbing or emitting a photon of frequency ω, if they possess an electric dipole moment p, oscillating at the same frequency Gross Selection Rule: A molecule has a rotational spectrum only if it has a permanent dipole moment. Rotating molecule _ _ + + t _ + _ + dipole momentp dipole Homonuclear molecules (e.g. O2, H2, Cl2, Br2…. do not have a permanent dipole moment and therefore do not have a microwave spectrum! General features of rotating systems m Linear velocity v angular velocity v = distance ω = radians O r time time v = ω × r Moment of Inertia I = mr2. A molecule can have three different moments of inertia IA, IB and IC about orthogonal axes a, b and c. 2 I = ∑miri i R Note how ri is defined, it is the perpendicular distance from axis of rotation ri Rigid Diatomic Rotors ro IB = Ic, and IA = 0. -

Laser Raman Spectroscopy As a Technique for Identification Of
ARTICLE IN PRESS CHEMGE-15589; No of Pages 13 Chemical Geology xxx (2008) xxx–xxx Contents lists available at ScienceDirect Chemical Geology journal homepage: www.elsevier.com/locate/chemgeo Laser Raman spectroscopy as a technique for identification of seafloor hydrothermal and cold seep minerals Sheri N. White ⁎ Department of Applied Ocean Physics and Engineering, Woods Hole Oceanographic Institution, Woods Hole, MA 02536, USA article info abstract Article history: In situ sensors capable of real-time measurements and analyses in the deep ocean are necessary to fulfill the Received 8 August 2008 potential created by the development of autonomous, deep-sea platforms such as autonomous and remotely Received in revised form 8 November 2008 operated vehicles, and cabled observatories. Laser Raman spectroscopy (a type of vibrational spectroscopy) is an Accepted 10 November 2008 optical technique that is capable of in situ molecular identification of minerals in the deep ocean. The goals of this Available online xxxx work are to determine the characteristic spectral bands and relative Raman scattering strength of hydrothermally- Editor: R.L. Rudnick and cold seep-relevant minerals, and to determine how the quality of the spectra are affected by changes in excitation wavelength and sampling optics. The information learned from this work will lead to the development Keywords: of new, smaller sea-going Raman instruments that are optimized to analyze minerals in the deep ocean. Raman spectroscopy Many minerals of interest at seafloor hydrothermal and cold seep sites are Raman active, such as elemental sulfur, Mineralogy carbonates, sulfates and sulfides. Elemental S8 sulfur is a strong Raman scatterer with dominant bands at ∼219 and Hydrothermal vents 472 Δcm−1. -

Photoionization Spectroscopy O
Photoionization spectroscopy of CH3C3N in the vacuum-ultraviolet range N. Lamarre, C. Falvo, C. Alcaraz, B. Cunha de Miranda, S. Douin, A. Flütsch, C. Romanzin, J.-C. Guillemin, Séverine Boyé-Péronne, B. Gans To cite this version: N. Lamarre, C. Falvo, C. Alcaraz, B. Cunha de Miranda, S. Douin, et al.. Photoionization spectroscopy of CH3C3N in the vacuum-ultraviolet range. Journal of Molecular Spectroscopy, Elsevier, 2015, 315, pp.206-216. 10.1016/j.jms.2015.03.005. hal-01138635 HAL Id: hal-01138635 https://hal-univ-rennes1.archives-ouvertes.fr/hal-01138635 Submitted on 4 Nov 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Photoionization spectroscopy of CH 3C3N in the vacuum-ultraviolet range N. Lamarre a, C. Falvo a, C. Alcaraz b,c, B. Cunha de Miranda b, S. Douin a, A. Fl utsch¨ a, C. Romanzin b, J.-C. Guillemin d, a, a, S. Boy e-P´ eronne´ ∗, B. Gans ∗ aInstitut des Sciences Mol´eculaires d’Orsay, Univ Paris-Sud; CNRS, bat 210, Univ Paris-Sud 91405 Orsay cedex (France) bLaboratoire de Chimie Physique, Univ Paris-Sud; CNRS UMR 8000, bat 350, Univ Paris-Sud 91405 Orsay cedex (France) cSynchrotron SOLEIL, L'Orme des Merisiers, St. -

Accessing Excited State Molecular Vibrations by Femtosecond Stimulated Raman Spectroscopy
Accessing Excited State Molecular Vibrations by Femtosecond Stimulated Raman Spectroscopy Giovanni Batignani,y Carino Ferrante,y,z and Tullio Scopigno∗,y,z yDipartimento di Fisica, Universitá di Roma “La Sapienza", Roma, I-00185, Italy z Istituto Italiano di Tecnologia, Center for Life Nano Science @Sapienza, Roma, I-00161, Italy E-mail: [email protected] arXiv:2010.05029v1 [physics.optics] 10 Oct 2020 1 Abstract Excited-state vibrations are crucial for determining photophysical and photochem- ical properties of molecular compounds. Stimulated Raman scattering can coherently stimulate and probe molecular vibrations with optical pulses, but it is generally re- stricted to ground state properties. Working in resonance conditions, indeed, enables cross-section enhancement and selective excitation to a targeted electronic level, but is hampered by an increased signal complexity due to the presence of overlapping spectral contributions. Here, we show how detailed information on ground and excited state vi- brations can be disentangled, by exploiting the relative time delay between Raman and probe pulses to control the excited state population, combined with a diagrammatic formalism to dissect the pathways concurring to the signal generation. The proposed method is then exploited to elucidate the vibrational properties of ground and excited electronic states in the paradigmatic case of Cresyl Violet. We anticipate that the presented approach holds the potential for selective mapping the reaction coordinates pertaining to transient electronic stages implied in photo-active compounds. Graphical TOC Entry 2 Raman spectroscopy is a powerful tool to access the vibrational fingerprints of molecules or solid state compounds and it can be used to extract structural and dynamical information of the samples under investigation. -

Absorption Spectroscopy and Imaging from the Visible Through Mid-Infrared with 20 Nm Resolution
Absorption spectroscopy and imaging from the visible through mid-infrared with 20 nm resolution. Aaron M. Katzenmeyer,1 Glenn Holland,1 Kevin Kjoller2 and Andrea Centrone1* 1Center for Nanoscale Science and Technology, National Institute of Standards and Technology, 100 Bureau Drive, Gaithersburg, Maryland 20899, United States. *E-mail: [email protected] 2Anasys Instruments, Inc., 325 Chapala Street, Santa Barbara, California 93101, United States. Abstract Absorption spectroscopy and mapping from visible through mid-IR wavelengths has been achieved with spatial resolution exceeding the limit imposed by diffraction, via the photothermal induced resonance technique. Correlated vibrational (chemical), and electronic properties are obtained simultaneously with topography with a wavelength-independent resolution of ≈ 20 nm using a single laboratory-scale instrument. This marks the highest resolution reported for PTIR, as determined by comparing height and PTIR images, and its first extension to near-IR and visible wavelengths. Light-matter interaction enables scientists to characterize materials and biological samples across a large range of energies. Visible (VIS) and near-infrared (NIR) light (from 400 nm to 2.5 µm) probes electronic transitions in materials [1], providing information regarding band gap, defects, and energy transfer which is crucial for the semiconductor and optoelectronic industries and for understanding processes such as photosynthesis. Mid-infrared (mid-IR) light (from 2.5 µm to 15 µm) probes vibrational transitions and provides rich chemical and structural information enabling materials identification [2]. Spectral maps can be obtained by coupling a light source and a spectrometer with an optical microscope [3]. However, light diffraction limits the lateral resolution achievable with conventional microscopic techniques to approximately half the wavelength of light [4]. -

Combining Chemical Information from Grass Pollen in Multimodal Characterization
ORIGINAL RESEARCH published: 31 January 2020 doi: 10.3389/fpls.2019.01788 Combining Chemical Information From Grass Pollen in Multimodal Characterization Sabrina Diehn 1,2, Boris Zimmermann 3, Valeria Tafintseva 3, Stephan Seifert 1,2, Murat Bag˘ cıog˘ lu 3, Mikael Ohlson 4, Steffen Weidner 2, Siri Fjellheim 5, Achim Kohler 3,6 and Janina Kneipp 1,2* 1 Department of Chemistry, Humboldt-Universität zu Berlin, Berlin, Germany, 2 BAM Federal Institute for Materials Research and Testing, Berlin, Germany, 3 Faculty of Science and Technology, Norwegian University of Life Sciences, Ås, Norway, 4 Faculty of Environmental Sciences and Natural Resource Management, Norwegian University of Life Sciences, Ås, Norway, 5 Faculty of Biosciences, Norwegian University of Life Sciences, Ås, Norway, 6 Nofima AS, Ås, Norway Edited by: Lisbeth Garbrecht Thygesen, fi University of Copenhagen, The analysis of pollen chemical composition is important to many elds, including Denmark agriculture, plant physiology, ecology, allergology, and climate studies. Here, the Reviewed by: potential of a combination of different spectroscopic and spectrometric methods Wesley Toby Fraser, regarding the characterization of small biochemical differences between pollen samples Oxford Brookes University, United Kingdom was evaluated using multivariate statistical approaches. Pollen samples, collected from Åsmund Rinnan, three populations of the grass Poa alpina, were analyzed using Fourier-transform infrared University of Copenhagen, Denmark (FTIR) spectroscopy, Raman spectroscopy, surface enhanced Raman scattering (SERS), Anna De Juan, and matrix assisted laser desorption/ionization mass spectrometry (MALDI-TOF MS). The University of Barcelona, Spain variation in the sample set can be described in a hierarchical framework comprising three *Correspondence: populations of the same grass species and four different growth conditions of the parent Janina Kneipp [email protected] plants for each of the populations. -

Hydrogen Conversion in Nanocages
Review Hydrogen Conversion in Nanocages Ernest Ilisca Laboratoire Matériaux et Phénomènes Quantiques, Université de Paris, CNRS, F-75013 Paris, France; [email protected] Abstract: Hydrogen molecules exist in the form of two distinct isomers that can be interconverted by physical catalysis. These ortho and para forms have different thermodynamical properties. Over the last century, the catalysts developed to convert hydrogen from one form to another, in laboratories and industries, were magnetic and the interpretations relied on magnetic dipolar interactions. The variety concentration of a sample and the conversion rates induced by a catalytic action were mostly measured by thermal methods related to the diffusion of the o-p reaction heat. At the turning of the new century, the nature of the studied catalysts and the type of measures and motivations completely changed. Catalysts investigated now are non-magnetic and new spectroscopic measurements have been developed. After a fast survey of the past studies, the review details the spectroscopic methods, emphasizing their originalities, performances and refinements: how Infra-Red measurements charac- terize the catalytic sites and follow the conversion in real-time, Ultra-Violet irradiations explore the electronic nature of the reaction and hyper-frequencies driving the nuclear spins. The new catalysts, metallic or insulating, are detailed to display the operating electronic structure. New electromagnetic mechanisms, involving energy and momenta transfers, are discovered providing a classification frame for the newly observed reactions. Keywords: molecular spectroscopy; nanocages; electronic excitations; nuclear magnetism Citation: Ilisca, E. Hydrogen Conversion in Nanocages. Hydrogen The intertwining of quantum, spectroscopic and thermodynamical properties of the 2021, 2, 160–206.