Biodiversity of Te Waikoropupü Springs Assessment and Vulnerabilities to Reduced Flows
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Zealand's Genetic Diversity
1.13 NEW ZEALAND’S GENETIC DIVERSITY NEW ZEALAND’S GENETIC DIVERSITY Dennis P. Gordon National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington 6022, New Zealand ABSTRACT: The known genetic diversity represented by the New Zealand biota is reviewed and summarised, largely based on a recently published New Zealand inventory of biodiversity. All kingdoms and eukaryote phyla are covered, updated to refl ect the latest phylogenetic view of Eukaryota. The total known biota comprises a nominal 57 406 species (c. 48 640 described). Subtraction of the 4889 naturalised-alien species gives a biota of 52 517 native species. A minimum (the status of a number of the unnamed species is uncertain) of 27 380 (52%) of these species are endemic (cf. 26% for Fungi, 38% for all marine species, 46% for marine Animalia, 68% for all Animalia, 78% for vascular plants and 91% for terrestrial Animalia). In passing, examples are given both of the roles of the major taxa in providing ecosystem services and of the use of genetic resources in the New Zealand economy. Key words: Animalia, Chromista, freshwater, Fungi, genetic diversity, marine, New Zealand, Prokaryota, Protozoa, terrestrial. INTRODUCTION Article 10b of the CBD calls for signatories to ‘Adopt The original brief for this chapter was to review New Zealand’s measures relating to the use of biological resources [i.e. genetic genetic resources. The OECD defi nition of genetic resources resources] to avoid or minimize adverse impacts on biological is ‘genetic material of plants, animals or micro-organisms of diversity [e.g. genetic diversity]’ (my parentheses). -

Torix Rickettsia Are Widespread in Arthropods and Reflect a Neglected Symbiosis
GigaScience, 10, 2021, 1–19 doi: 10.1093/gigascience/giab021 RESEARCH RESEARCH Torix Rickettsia are widespread in arthropods and Downloaded from https://academic.oup.com/gigascience/article/10/3/giab021/6187866 by guest on 05 August 2021 reflect a neglected symbiosis Jack Pilgrim 1,*, Panupong Thongprem 1, Helen R. Davison 1, Stefanos Siozios 1, Matthew Baylis1,2, Evgeny V. Zakharov3, Sujeevan Ratnasingham 3, Jeremy R. deWaard3, Craig R. Macadam4,M. Alex Smith5 and Gregory D. D. Hurst 1 1Institute of Infection, Veterinary and Ecological Sciences, Faculty of Health and Life Sciences, University of Liverpool, Leahurst Campus, Chester High Road, Neston, Wirral CH64 7TE, UK; 2Health Protection Research Unit in Emerging and Zoonotic Infections, University of Liverpool, 8 West Derby Street, Liverpool L69 7BE, UK; 3Centre for Biodiversity Genomics, University of Guelph, 50 Stone Road East, Guelph, Ontario N1G2W1, Canada; 4Buglife – The Invertebrate Conservation Trust, Balallan House, 24 Allan Park, Stirling FK8 2QG, UK and 5Department of Integrative Biology, University of Guelph, Summerlee Science Complex, Guelph, Ontario N1G 2W1, Canada ∗Correspondence address. Jack Pilgrim, Institute of Infection, Veterinary and Ecological Sciences, Faculty of Health and Life Sciences, University of Liverpool, Liverpool, UK. E-mail: [email protected] http://orcid.org/0000-0002-2941-1482 Abstract Background: Rickettsia are intracellular bacteria best known as the causative agents of human and animal diseases. Although these medically important Rickettsia are often transmitted via haematophagous arthropods, other Rickettsia, such as those in the Torix group, appear to reside exclusively in invertebrates and protists with no secondary vertebrate host. Importantly, little is known about the diversity or host range of Torix group Rickettsia. -

A Directory of Wetlands in New Zealand: Nelson/Marlborough
A Directory of Wetlands in New Zealand NELSON/MARLBOROUGH CONSERVANCY Farewell Spit (39) Location: 40o32'S, 172o50'E. At the northern extremity of Golden Bay and the northwestern extremity of South Island, 38 km from the town of Takaka, Tasman District. Area: 11,388 ha (land area c.1,961 ha; inter-tidal zone c.9,427 ha). Altitude: Sea level to 3 m. - 155 - A Directory of Wetlands in New Zealand Overview: Farewell Spit is a classic recurved spit, approximately 30 km long, composed predominantly of uniform quartz sand derived from rivers draining westwards and transported northward by the westland current. The north is exposed to the Tasman Sea, but the south has extensive tidal mudflats. These provide feeding areas for large numbers of waterfowl. Some 95 species were recorded on the spit in March 1974, and more than 83 species of wetland birds are regularly recorded at the spit. The sand dunes provide habitat for a diverse and unusual plant community. Farewell Spit was listed as a wetland of international importance under the Ramsar Convention on 13 August 1976. Physical features: Farewell Spit is a classic recurved spit. The material forming the spit is derived from erosion of the Southern Alps and West Coast sea cliffs, transported northwards by a long-shore current. Since the estimated origin of the spit 6,500 years ago, an estimated 2.2 million cubic metres of sand have been deposited per annum. Wind transports more surface sand towards Golden Bay, although the majority of sand lies below the mean low water mark. -

New Zealand 16 Marlborough Nelson Chapter
©Lonely Planet Publications Pty Ltd Marlborough & Nelson Why Go? Marlborough Region ....400 For many travellers, Marlborough and Nelson will be their Picton ........................... 400 introduction to what South Islanders refer to as the ‘Main- Marlborough Sounds ...404 land’. Having left windy Wellington, and made a white- Queen Charlotte Track ...407 knuckled crossing of Cook Strait, folk are often surprised to fi nd the sun shining and the temperature up to 10 degrees Kenepuru & Pelorus Sounds.............409 warmer. Good pals, these two neighbouring regions have much Blenheim ........................411 in common beyond an amenable climate: both boast re- Kaikoura ........................ 416 nowned coastal holiday spots, particularly the Marlborough Nelson ...........................423 Sounds and Abel Tasman National Park. There are two other Nelson Lakes national parks (Kahurangi and Nelson Lakes) and more National Park ................430 mountain ranges than you can poke a stick at. Motueka ........................432 And so it follows that these two regions have an abun- Motueka to Abel dance of luscious produce: summer cherries for a starter, Tasman ..........................435 but most famously the grapes that work their way into the Golden Bay ....................440 wineglasses of the world’s fi nest restaurants. Keep your pen- Kahurangi National knife and picnic set at the ready. Park ...............................444 When to Go? Best Places to Eat The forecast is good: Marlborough and Nelson soak up some » Green Dolphin (p 422 ) of New Zealand’s sunniest weather. January and February are the warmest months, with daytime temperatures aver- » Wither Hills (p 414 ) aging 22°C; July is the coldest, averaging 12°C. It’s wetter » Hopgood’s (p 428 ) and more windswept the closer you get to Farewell Spit and » Sans Souci Inn (p 442 ) the West Coast. -

For More Information on the Abel Tasman National Park Please Visit: Wild About New Zealand Travel Information Web Site
WILD ABOUT NEW ZEALAND - ABEL TASMAN & NELSON LAKES NATIONAL PARK EPISODE GUIDE TO 10/9/13 Episode Two: Abel Tasman – Nelson Lakes National Park, Wild About New Zealand Series. Aired: Tuesday, September 10th, 8.30 on TV ONE By Gus Roxburgh The Wild About New Zealand Episode guides provide a simple overview for viewers on planning a trip to the National Park featured in the TV series. What can you visit? What is easily accessible? What needs more planning and preparation? Plus top tips on things to do in and around the National Parks. The guides are written by series presenter, Gus Roxburgh who spent 18 months on adventures – both big and small, in all our National Parks featured in the series. The information is designed to get New Zealanders to move from the inspiration of the TV series to action - and head out to explore our world renowned National Parks. Gus is presenter of the TV series, Wild About New Zealand, and principal author of the book of the series being published by Random House in October 2013 Content developed with Nelson and Tasman Regional Tourism Office, Department of Conservation and Jasons Travel Media. For more information on the Abel Tasman National Park please visit: Wild About New Zealand Travel Information Web Site: www.wildaboutnewzealand.co.nz 1 | P a g e Wild About New Zealand Episode Guide WILD ABOUT NEW ZEALAND - ABEL TASMAN & NELSON LAKES NATIONAL PARK EPISODE GUIDE TO 10/9/13 Episode Guide Sections : i. Essential Travel Information incl. Why You Should Visit, What is So Special, Getting There & 5 Top Things to Do ii. -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000). -

Figueroa Et Al Chile Mediterranean Re-Submitted
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Diposit Digital de la Universitat de Barcelona 1 Freshwater biodiversity and conservation in mediterranean climate streams of 2 Chile 3 4 1 Ricardo Figueroa* 5 2 Núria Bonada 6 1 Meyer Guevara 7 1 Pablo Pedreros 8 1 Francisco Correa-Araneda 9 1 María E. Díaz 10 3 Victor H. Ruiz 11 12 *1Corresponding author: [email protected] Center of Environmental Sciences EULA- 13 Chile, University of Concepcion, Casilla 160-C, Concepcion, Chile 14 2Grup de Recerca Freshwater Ecology and Management (FEM), Departament 15 d’Ecologia, Facultat de Biologia, Universitat de Barcelona (UB), Diagonal 643, 08028 16 Barcelona, Catalonia/Spain 17 3Department of Zoology, University of Concepcion, P.O. Box 160-C, Concepción, 18 Chile 19 20 21 Keywords: Central Chile, diversity, endemism, fauna, flora, streams and rivers 22 23 1 24 Abstract 25 26 In Chile, mediterranean climate conditions only occur in the Central Zone (ChMZ). 27 Despite its small area, this mediterranean climate region (med-region) has been 28 recognised as a hotspot for biodiversity. However, in contrast to the rivers of other med- 29 regions, the rivers in the ChMZ have been studied infrequently, and knowledge of their 30 freshwater biodiversity is scarce and fragmented. We gathered information on the 31 freshwater biodiversity of ChMZ, and present a review of the current knowledge of the 32 principal floral and faunal groups. Existing knowledge indicates that the ChMZ has high 33 levels of endemism, with many primitive species being of Gondwanan origin. -
Dump Station in New Zealand a Guide for Motor Home Users Motor Home Users
FINDING A DUMP STATION IN NEW ZEALAND A GUIDE FOR MOTOR HOME USERS MOTOR HOME USERS Be responsible when emptying your motor home toilet and waste water – always use a designated dump station. It’s better for New Zealand’s environment and our health. Play your part and help look after our environment. You can find your nearest dump station by using this guide, and looking for the blue symbol. DUMP STATIONS AT HOLIDAY PARKS Dump stations at holiday parks are for guests only. There may be a charge at some holiday parks for use by non-guests. Key DOC Department of Conservation Mt Mount RD Rural Delivery SH State Highway For extra information visit the Ministry of Tourism’s website www.tourism.govt.nz 1. NORTHLAND Haruru Falls, ‘Panorama’ Old Wharf Road, Haruru Falls The Park Top 10 Ninety Mile Beach Ninety Mile Beach, Kaitaia, Twin Pines Tourist Park 18 km north, Kaitaia, Uri ramp Puketona Road, Haruru Falls, Paihia Bay of Islands Holiday Park Whatuwhiwhi Top 10 Holiday Park Lily Pond, Puketona Road, Paihia 17 Whatuwhiwhi Road, Kaitaia Beachside Holiday Park Wagener Holiday Park Paihia-Opua Road, Paihia Houhora Heads, Kaitaia Russell Top 10 Holiday Park Kaitaia Public Dump Station Long Beach Road, Russell Located behind Community Centre, junction of Mathews Ave and SH!, Waitangi Holiday Park Kaitaia 21 Tahuna Road, Waitangi Mangonui Public Dump Station Oakura Motels & Caravan Park Beach Road, next to public toilets, Te Kapua Street, Oakura Bay 400km from SH10, Kaitaia Kawakawa Public Dump Station Norfolk Campervan Park Waimio Street, off SH!, -

New Zealand Freshwater Sciences Society Newsletter Number 41
New Zealand Freshwater Sciences Society Newsletter Number 41 November 2005 New Zealand Freshwater Sciences Society Newsletter No. 41 November 2005 Contents Introduction to the Society ....................................................................... 3 Editorial ............................................................................................. 4 President’s comment ................................................................................ 5 Research news ...................................................................................... 6 Freshwater Sciences Society Conference, Rotorua, 26 – 30 November 2006 ...............34 S.I.L. 1987 Trust Fund Awards .................................................................35 New Zealand Freshwater Sciences Society Medal and Honorary Membership ...............38 Minutes of the 37th Annual General Meeting ....................................................39 of the New Zealand Limnological Society Inc. (2004) .........................................39 Conference prizes 2004...........................................................................47 Conference Prizes, 2005..........................................................................48 Special Publications................................................................................50 Publications and theses ...........................................................................50 Membership list....................................................................................61 How do I join?.....................................................................................78 -

Annotated Checklist of New Zealand Decapoda (Arthropoda: Crustacea)
Tuhinga 22: 171–272 Copyright © Museum of New Zealand Te Papa Tongarewa (2011) Annotated checklist of New Zealand Decapoda (Arthropoda: Crustacea) John C. Yaldwyn† and W. Richard Webber* † Research Associate, Museum of New Zealand Te Papa Tongarewa. Deceased October 2005 * Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, New Zealand ([email protected]) (Manuscript completed for publication by second author) ABSTRACT: A checklist of the Recent Decapoda (shrimps, prawns, lobsters, crayfish and crabs) of the New Zealand region is given. It includes 488 named species in 90 families, with 153 (31%) of the species considered endemic. References to New Zealand records and other significant references are given for all species previously recorded from New Zealand. The location of New Zealand material is given for a number of species first recorded in the New Zealand Inventory of Biodiversity but with no further data. Information on geographical distribution, habitat range and, in some cases, depth range and colour are given for each species. KEYWORDS: Decapoda, New Zealand, checklist, annotated checklist, shrimp, prawn, lobster, crab. Contents Introduction Methods Checklist of New Zealand Decapoda Suborder DENDROBRANCHIATA Bate, 1888 ..................................... 178 Superfamily PENAEOIDEA Rafinesque, 1815.............................. 178 Family ARISTEIDAE Wood-Mason & Alcock, 1891..................... 178 Family BENTHESICYMIDAE Wood-Mason & Alcock, 1891 .......... 180 Family PENAEIDAE Rafinesque, 1815 .................................. -
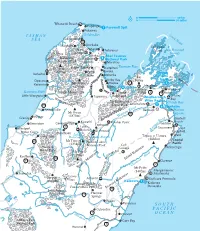
P a C I F I C O C E a N S O U
050 km 0 30 miles Wharariki Beach Puponga Farewell Spit Pakawau T A S M A N Golden Bay Cook Strait S E A Collingwood 60 Onekaka Bainham D'Urville Pupu Pohara Totaranui Island Marlborough y Trac Springs Sounds h k Takaka ap Abel Tasman e Kahurangi H Canaan National Park National Park Downs Marahau Tasman Bay Mt Domett Upper Takaka Kaiteriteri (1646m) Cobb Riwaka Queen y Ferry to Kohaihai River Motueka w Rai Charlotte Wellington H Moutere Oparara Ruby Bay Valley Track Mt Arthur Valley Mapua 6 Karamea Havelock (1795m) Rabbit Nelson ueka Hwy Is Pelorus Waikawa 6 Karamea Bight Tasman Mountains Stoke Bridge 1 Picton Mot Little Wanganui Tapawera Richmond Marlborough Whites Wangape Bay Mt Richmond Wine Region ka ck Cloudy Bay 67 Tra Forest Park Onamalutu er Blenheim Seddonville ge iv Wairau an u R Renwick Mt Owen d R ira 65 Lagoons (1875m) on Wa Granity Hector ichm r R ive Marfells Kawatiri Kowhai Point R Beach Denniston Gowanbridge Tophouse Seddon 6 Lake Westport Grassmere Cape Rotoroa St Arnaud Awatere Campbell Buller Gorge Murchison Lake Ward Inangahua Rotoroa Lake Rotoiti Tapuae-o-Uenuru (2885m) Coastal 69 Mt Travers Nelson Lakes Pacific (2338m) National Park Cob Victoria Cottage ras Kekerengu Forest Park ou Mt Una Rd aik (2300m) eron d K Reefton ch River 65 Inlan 7 -A Clarence rth l) 1 o Clarence Spencer Mountains na Mt Fyffe Mangamaunu; lesw aso (1402m) Ikamatua o e Meatworks (S Lewis M Springs Acheron Kaikoura Peninsula Junction Pass KaikourasKaikoura Hanmer Forest ardConway Riv Kaikoura Peninsula Conservation Park Seaw70 Lake Sumner Forest Park 7 Hanmer iver Springs R er Waiau Waiau Parnassus S O U T H Culverden P A C I F I C 7 O C E A N Huru Cheviot nui r Arthur's Pass Rive Gore Bay National Park 1 Hurunui. -

Observations on the Food of Freshwater Fish from the Coal and Jordan Rivers, Tasmania
https://doi.org/10.26749/rstpp.111.59 Papers and Proceedings of the RoyaJ Society of Tasmania, Volume 111, 1977 (ms. received 22.]2.1976) OBSERVATIONS ON THE FOOD OF FRESHWATER FISH FROM THE COAL AND JORDAN RIVERS, TASMANIA hy P. S. Lakea and G. Department of Zoology, University of Tasmania (a) now Department of Zoology, Monash University, Clayton, Victoria (b) now 4 Fellows Road, Hughesdale, Victoria (with five tables) ABSTRACT The Coal and the Jordan Rivers are two slow-flowing rivers in south-eastern Tasmania. Details are given of the stomach contents of brown trout Salmo trutta Linnaeus, English perch Perea fZuviati lis Linnaeus, short-finned eels australis oeeidentaZ-is Schmidt and Tasmanian smelt Retrop-i-nna tasmaniea McCulloch collected from the Coal River and of the stomach contents of brown trout, English perch, tench, short-finned eels, freshwater flathead Pseudaphritis urvi ZZi (Cuvier and Valenciennes) and galaxiids GaZax1:as maeuZatus (Jenyns) collected from the Jordan River. INTRODUCTION The food of trout in Australia has been studied by McKeown (1934a, 1934b, 1936, 1937, 1955), Evans (1942), Butcher (1945, 1946), Wilson (1966) and Knott (1973). However, there are few published data on the food of native freshwater fish or even of non-salmonid introduced fish, Butcher (1945, 1946) examined the food of perch, austraZasica (Cuvier & Valenciennes), English marmoratus (Richardson) and (.Jcnyns). food of six Macquarie perch and brief non-quantitative the food of some native and introduced freshwater fish of New South Wales are given by Lake (1959). Recently Pollard (1973) has provided a detailed account of the diet of land-locked Gal-axias macuZatus in Lake ModevJarre, Victoria.