Unique Meteorite from Early Amazonian Mars
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shergotty Basalt, 5 Kg Seen to Fall Introduction the Shergotty Achondrite Fell on August 25, 1865 at 9:00 A.M
V. Shergotty basalt, 5 kg seen to fall Introduction The Shergotty achondrite fell on August 25, 1865 at 9:00 a.m. near a town called Shergahti in Bihar State, India after detonations were heard (Graham et al. 1985). Duke (1968) refers to several stones with fusion crusts, but this has not been confirmed. The main mass is at the Museum of the Geological Survey in Calcutta, India (figure V-1). In 1984, an international consortium was organized by J. C. Laul to study ~30 grams of Shergotty in detail (Laul 1986a, b). Shergottites (Shergotty, Zagami, EETA79001B, QUE94201, Los Angeles) are texturally and mineralogically similar to terrestrial diabases (although all of the plagioclase has been shocked to maskelynite), but quite distinct petrologically and chemically from the rest of the basaltic achondrites (Stolper et al. 1979). Stolper and McSween (1979) and others have noted that Shergotty crystallized under relatively oxidizing conditions. Figure V-1. Photograph of Shergotty meteorite The Shergotty meteorite has been severely shocked and showing fusion crust and borken surfaces. Two saw is considered the “type locality” for maskelynite (dense cuts are visible. Sample is about 25 cm across. Photo plagioclase glass). In fact, it has proven to be very kindly provided by Prof. N. Bhandari, Director, Physical Research Laboratory, Ahmedabad, India. difficult to date the original crystallization event of Figure V-2. Photograph of slab of Shergotty meteorite showing basaltic texture and alignment of pyroxene crystals. Note the inclusion of black glass in the center of this slab. This is figure 1 in Duke (1968). Photo courtesy of U. -

LEW 88516.Pmd
LEW88516 – 13.2 grams Lherzolitic Shergottite Figure 1. Photograph of LEW88516 after first break for processing. Cube is 1 cm for scale. NASA # S91-37060 Introduction meteorite has three distinct textural regions: 1) poikilitic Lewis Cliff sample LEW88516 is petrologically similar crystalline, 2) interstitial crystalline (or non-poikilitic) to ALH77005 (Satterwhite and Mason 1991; Treiman and, 3) glassy to partially crystalline veinlets. Delaney et al. 1994) and Y793605 (Kojima et al. 1997). (1992), Lindstrom et al. (1992) and Harvey and However, it is not paired, because it has a distinctly McSween (1992) have also reported petrological different terrestrial exposure age. Figure 1 shows descriptions of LEW88516. Papike et al. (2009) LEW88516 as a small (2 cm), dark, rounded, meteorite compared LEW88516 with the rest of the shergottites. with coarsely crystalline interior. In the poikilitic regions, mm-sized olivine crystals and LEW88516 was a small nondescript meteorite that was 50 micron-sized chromite crystals are contained within, not recognized as an achondrite until it was broken open and completely surrounded by, pigeonite crystals with Hence, it waited 2 years to be processed in numerical exsolution lamellae of augite. Maskelynite, whitlockite order. According to Satterwhite and Mason (1991), and sulfides are rare in the poikilitic regions. LEW88516 had a “pitted and mostly shiny fusion crust over 80 % of the surface.” Preliminary The interstitial areas have a more typically basaltic examination of the thin section showed about 50 % texture with intergrown euhedral and subhedral olivine, olivine, 35 % pyroxene, 8 % interstitial maskelynite with pigeonite and chromite, with interstitial maskelynite, about 5% brown glass. Grain size is about 2 - 3 mm. -
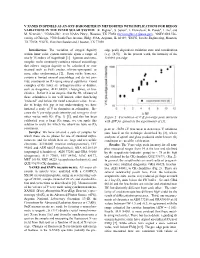
V Xanes in Spinels As an Oxy-Barometer in Meteorites with Implications for Redox Variations in the Inner Solar System
V XANES IN SPINELS AS AN OXY-BAROMETER IN METEORITES WITH IMPLICATIONS FOR REDOX VARIATIONS IN THE INNER SOLAR SYSTEM. K. Righter1, S. Sutton2, L. Danielson3, K. Pando4, L. Le3, and M. Newville2. 1NASA-JSC, 2101 NASA Pkwy., Houston, TX 77058 ([email protected]), 2GSECARS Uni- versity of Chicago, 9700 South Cass Avenue, Bldg. 434A, Argonne, IL 60439; 3ESCG, Jacobs Engineering, Houston, TX 77058; 4ESCG, Hamilton Sundstrand, Houston, TX 77058 Introduction: The variation of oxygen fugacity edge peak) depend on oxidation state and coordination within inner solar system materials spans a range of (e.g., [4,5]). In the present work, the intensity of the nearly 15 orders of magnitiude [1]. Igneous and meta- XANES pre-edge morphic rocks commonly contain a mineral assemblage that allows oxygen fugacity to be calculated or con- strained such as FeTi oxides, olivine-opx-spinel, or some other oxybarometer [2]. Some rocks, however, contain a limited mineral assemblage and do not pro- vide constraints on fO2 using mineral equilibria. Good examples of the latter are orthopyroxenites or dunites, such as diogenites, ALH 84001, chassignites, or bra- chinites. In fact it is no surprise that the fO2 of many of these achondrites is not well known, other than being "reduced" and below the metal saturation value. In or- der to bridge this gap in our understanding, we have initiated a study of V in chromites in achondrite. Be- cause the V pre-edge peak intensity and energy in chro- mites varies with fO2 (Fig. 1) [3], and this has been Figure 1: Correlation of V K pre-edge peak intensity calibrated over a large fO2 range, we can apply this with IW for spinels in the experiments of [3]. -

Linking the Chassigny Meteorite and the Martian Surface Rock Backstay: Insights Into Igneous Crustal Differentiation Processes on Mars
Meteoritics & Planetary Science 44, Nr 6, 853–869 (2009) Abstract available online at http://meteoritics.org Linking the Chassigny meteorite and the Martian surface rock Backstay: Insights into igneous crustal differentiation processes on Mars Hanna NEKVASIL*, Francis M. MCCUBBIN, Andrea HARRINGTON, Stephen ELARDO, and Donald H. LINDSLEY Department of Geosciences, Stony Brook University, Stony Brook, New York 11794–2100, USA *Corresponding author. E-mail: [email protected] (Received 05 August 2008; revision accepted 18 March 2009) Abstract–In order to use igneous surface lithologies to constrain Martian mantle characteristics, secondary processes that lead to compositional modification of primary mantle melts must be considered. Crystal fractionation of a mantle-derived magma at the base of the crust followed by separation and ascent of residual liquids to the surface is common in continental hotspot regions on Earth. The possibility that this process also takes place on Mars was investigated by experimentally determining whether a surface rock, specifically the hawaiite Backstay analyzed by the MER Spirit could produce a known cumulate lithology with a deep origin (namely the assemblages of the Chassigny meteorite) if trapped at the base of the Martian crust. Both the major cumulus and melt inclusion mineral assemblages of the Chassigny meteorite were produced experimentally by a liquid of Backstay composition within the pressure range 9.3 to 6.8 kbar with bulk water contents between 1.5 and 2.6 wt%. Experiments at 4.3 and 2.8 kbar did not produce the requisite assemblages. This agreement suggests that just as on Earth, Martian mantle-derived melts may rise to the surface or remain trapped at the base of the crust, fractionate, and lose their residual liquids. -

Assessment of the Mesosiderite-Diogenite Connection and an Impact Model for the Genesis of Mesosiderites
45th Lunar and Planetary Science Conference (2014) 2554.pdf ASSESSMENT OF THE MESOSIDERITE-DIOGENITE CONNECTION AND AN IMPACT MODEL FOR THE GENESIS OF MESOSIDERITES. T. E. Bunch1,3, A. J. Irving2,3, P. H. Schultz4, J. H. Wittke1, S. M. Ku- ehner2, J. I. Goldstein5 and P. P. Sipiera3,6 1Dept. of Geology, SESES, Northern Arizona University, Flagstaff, AZ 86011 ([email protected]), 2Dept. of Earth & Space Sciences, University of Washington, Seattle, WA, 3Planetary Studies Foundation, Galena, IL, 4Dept. of Geological Sciences, Brown University, Providence, RI, 5Dept. of Geolo- gy, University of Massachusetts, Amherts, MA, 6Field Museum of Natural History, Chicago, IL. Introduction: Among well-recognized meteorite 34) is the most abundant silicate mineral and in some classes, the mesosiderites are perhaps the most com- clasts contains inclusions of FeS, tetrataenite, merrillite plex and petrogenetically least understood. Previous and silica. Three of the ten norite clasts contain a few workers have contributed important information about tiny grains of olivine (Fa24-32). A single, fine-grained “classic” falls and Antarctic finds, and have proposed breccia clast was found in NWA 5312 (see Figure 2). several different models for mesosiderite genesis [1]. Unlike the case of pallasites, the co-occurrence of met- al and silicates (predominantly orthopyroxene and cal- cic plagioclase) in mesosiderites is inconsistent with a single-stage “igneous” history, and instead seems to demand admixture of at least two separate compo- nents. Here we review the models in light of detailed ex- amination of multiple specimens from a very large mesosiderite strewnfield in Northwest Africa. Many specimens (totaling at least 80 kilograms) from this area (probably in Algeria) have been classified sepa- rately by us and others; however, in most cases the Figure 1. -

SPECTRAL CHARACTERIZATION of the ANCIENT SHERGOTTITES NORTHWEST AFRICA 7034 and 8159. KJ Orr1, LV Forman1, GK Benedix1
Ninth International Conference on Mars 2019 (LPI Contrib. No. 2089) 6177.pdf SPECTRAL CHARACTERIZATION OF THE ANCIENT SHERGOTTITES NORTHWEST AFRICA 7034 AND 8159. K. J. Orr1, L. V. Forman1, G. K. Benedix1, M. J. Hackett2, V. E. Hamilton3, and A. R. Santos. 1Space Science and Technology Centre (SSTC), School of Earth and Planetary Sciences, Curtin University, Perth, Western Australia, Australia ([email protected]), 2School of Molecular and Life Sciences, Curtin University, Perth, Western Australia, Australia, 3Southwest Research Institute, 1050 Walnut St. #300, Boulder, CO 80302 USA. 4USRA, 7178 Columbia Gateway Dr., Columbia, MD 21046. Introduction: Thermal infrared (TIR) spectros- Ga), the oldest confirmed shergottites recovered so far copy is a powerful remote sensing tool used to unravel [5]. As the only shergottites of Early Amazonian to No- the surface compositions of a target body. This tech- achian in age, they provide an invaluable opportunity to nique has been widely used in space missions, because understanding Mars’ early history. of its ability to detect and determine modal mineralogy Methods: Both samples (~0.5g chips) were ac- of the surface geology. It has been instrumental in de- quired from UNM and were made into epoxy mounts. veloping our understanding of Mars, as the majority of NWA 8159 was analyzed with a Tescan Integrated Min- missions sent to Mars have included an infrared spec- eral Analyzer (TIMA) to determine modal mineral trometer. These spectrometers can operate either in the abundancies and produce high-resolution mineral maps. visible (VIS) to near-infrared (NIR) or in the mid-infra- NWA 8159 was also analyzed using EBSD to charac- red (MIR). -

Validity of the Apatite/Merrillite Relationship in Evaluating the Water Content in the Martian Mantle: Implications from Shergottite Northwest Africa (NWA) 2975
Originally published as: Słaby, E., Förster, H.‐J., Wirth, R., Giera, A., Birski, Ł., Moszumańska, I. (2017): Validity of the Apatite/Merrillite Relationship in Evaluating the Water Content in the Martian Mantle: Implications from Shergottite Northwest Africa (NWA) 2975. ‐ Geosciences, 7, 4. DOI: http://doi.org/10.3390/geosciences7040099 geosciences Article Validity of the Apatite/Merrillite Relationship in Evaluating the Water Content in the Martian Mantle: Implications from Shergottite Northwest Africa (NWA) 2975 Ewa Słaby 1,*, Hans-Jürgen Förster 2, Richard Wirth 2, Alicja Wudarska 1,2, Łukasz Birski 1 and Izabela Moszuma ´nska 1 1 Institute of Geological Sciences, Polish Academy of Sciences, Research Centre in Warsaw, Twarda 51/55, 00-818 Warsaw, Poland; [email protected] (A.W.); [email protected] (Ł.B.); [email protected] (I.M.) 2 Helmholtz-Zentrum Potsdam Deutsches GeoForschungsZentrum GFZ, Telegrafenberg, 14473 Potsdam, Germany; [email protected] (H.-J.F.); [email protected] (R.W.) * Correspondence: [email protected] Received: 17 July 2017; Accepted: 26 September 2017; Published: 4 October 2017 Abstract: Phosphates from the Martian shergottite NWA 2975 were used to obtain insights into the source and subsequence differentiation of the melt/melts. The crystallization of two generations of fluorapatite (F > Cl~OH and F-rich), chlorapatite and ferromerrillite-merrillite were reconstructed from TEM (Transmission Electron Microscopy) and geochemical analyses. The research results indicated that the recognized volatiles budget of the two generations of fluorapatite was related to their magmatic origin. The apatite crystals crystallized from an evolved magma during its final differentiation and degassing stage. -

“Mining” Water Ice on Mars an Assessment of ISRU Options in Support of Future Human Missions
National Aeronautics and Space Administration “Mining” Water Ice on Mars An Assessment of ISRU Options in Support of Future Human Missions Stephen Hoffman, Alida Andrews, Kevin Watts July 2016 Agenda • Introduction • What kind of water ice are we talking about • Options for accessing the water ice • Drilling Options • “Mining” Options • EMC scenario and requirements • Recommendations and future work Acknowledgement • The authors of this report learned much during the process of researching the technologies and operations associated with drilling into icy deposits and extract water from those deposits. We would like to acknowledge the support and advice provided by the following individuals and their organizations: – Brian Glass, PhD, NASA Ames Research Center – Robert Haehnel, PhD, U.S. Army Corps of Engineers/Cold Regions Research and Engineering Laboratory – Patrick Haggerty, National Science Foundation/Geosciences/Polar Programs – Jennifer Mercer, PhD, National Science Foundation/Geosciences/Polar Programs – Frank Rack, PhD, University of Nebraska-Lincoln – Jason Weale, U.S. Army Corps of Engineers/Cold Regions Research and Engineering Laboratory Mining Water Ice on Mars INTRODUCTION Background • Addendum to M-WIP study, addressing one of the areas not fully covered in this report: accessing and mining water ice if it is present in certain glacier-like forms – The M-WIP report is available at http://mepag.nasa.gov/reports.cfm • The First Landing Site/Exploration Zone Workshop for Human Missions to Mars (October 2015) set the target -

Physical Properties of Martian Meteorites: Porosity and Density Measurements
Meteoritics & Planetary Science 42, Nr 12, 2043–2054 (2007) Abstract available online at http://meteoritics.org Physical properties of Martian meteorites: Porosity and density measurements Ian M. COULSON1, 2*, Martin BEECH3, and Wenshuang NIE3 1Solid Earth Studies Laboratory (SESL), Department of Geology, University of Regina, Regina, Saskatchewan S4S 0A2, Canada 2Institut für Geowissenschaften, Universität Tübingen, 72074 Tübingen, Germany 3Campion College, University of Regina, Regina, Saskatchewan S4S 0A2, Canada *Corresponding author. E-mail: [email protected] (Received 11 September 2006; revision accepted 06 June 2007) Abstract–Martian meteorites are fragments of the Martian crust. These samples represent igneous rocks, much like basalt. As such, many laboratory techniques designed for the study of Earth materials have been applied to these meteorites. Despite numerous studies of Martian meteorites, little data exists on their basic structural characteristics, such as porosity or density, information that is important in interpreting their origin, shock modification, and cosmic ray exposure history. Analysis of these meteorites provides both insight into the various lithologies present as well as the impact history of the planet’s surface. We present new data relating to the physical characteristics of twelve Martian meteorites. Porosity was determined via a combination of scanning electron microscope (SEM) imagery/image analysis and helium pycnometry, coupled with a modified Archimedean method for bulk density measurements. Our results show a range in porosity and density values and that porosity tends to increase toward the edge of the sample. Preliminary interpretation of the data demonstrates good agreement between porosity measured at 100× and 300× magnification for the shergottite group, while others exhibit more variability. -

MARTIAN METEORITES: GEOCHEMISTRY and SUCH 6:00 P.M
Lunar and Planetary Science XLVIII (2017) sess333.pdf Tuesday, March 21, 2017 [T333] POSTER SESSION I: MARTIAN METEORITES: GEOCHEMISTRY AND SUCH 6:00 p.m. Town Center Exhibit Area Irving A. J. Kuehner S. M. Lapen T. J. Righter M. Busemann H. et al. POSTER LOCATION #466 Keeping Up with the Martian Meteorites and Constraining the Number of Separate Launch Sites on Mars [#2068] Petrologic, chemical, and cosmogenic nuclide criteria suggest that the 101 unpaired martian meteorites may come from as few as 20 launch sites on Mars. Habermann M. Agee C. Spilde M. POSTER LOCATION #467 Multi-Layered Clast in Martian Breccia Northwest Africa 10922 [#2743] We performed chemical analyses of an unusual, concentrically-layered clast within the martian breccia NWA 10922. Santos A. R. Lewis J. A. Agee C. B. Humayun M. McCubbin F. M. et al. POSTER LOCATION #468 Feldspar Variability in Northwest Africa 7034 [#2349] Northwest Africa 7034 contains feldspar of different grain size, texture, and composition, some of which suggest modification by secondary alteration. Cao T. He Qi. POSTER LOCATION #469 Petrology and Mineral Chemistry of the Eniched Basaltic Shergottite Northwest Africa 8656 [#1384] The characterization of primary petrography and mineralogy of Northwest Africa 8656, basalt shergottite, are described in this abstract. Jacobs G. M. Abernethey F. J. Anand M. Franchi I. A. Grady M. M. POSTER LOCATION #470 Understanding the Early Martian Environment Through the Inventory of Breccia Clasts in Northwest Africa 7034 [#2139] The clasts and matrices of Northwest Africa 7034 and its breccia clasts reveal the meteorite aggregates material from multiple sources with distinct histories. -

AST-2018-1847-Ver9-Hughes 4P 260..283
ASTROBIOLOGY Volume 19, Number 3, 2019 Mary Ann Liebert, Inc. DOI: 10.1089/ast.2018.1847 Basaltic Terrains in Idaho and Hawai‘i as Planetary Analogs for Mars Geology and Astrobiology Scott S. Hughes,1 Christopher W. Haberle,2 Shannon E. Kobs Nawotniak,1 Alexander Sehlke,3 W. Brent Garry,4 Richard C. Elphic,3 Samuel J. Payler,5 Adam H. Stevens,5 Charles S. Cockell,5 Allyson L. Brady,6 Jennifer L. Heldmann,3,7 and Darlene S.S. Lim3,8 Abstract Field research target regions within two basaltic geologic provinces are described as Earth analogs to Mars. Regions within the eastern Snake River Plain of Idaho and the Big Island of Hawai‘i, the United States, provinces that represent analogs of present-day and early Mars, respectively, were evaluated on the basis of geologic settings, rock lithology and geochemistry, rock alteration, and climate. Each of these factors provides rationale for the selection of specific targets for field research in five analog target regions: (1) Big Craters and (2) Highway lava flows at Craters of the Moon National Monument and Preserve, Idaho, and (3) Mauna Ulu low shield, (4) Kılauea Iki lava lake, and (5) Kılauea caldera in the Kılauea Volcano summit region and the East Rift Zone of Hawai‘i. Our evaluation of compositional and textural attributes, as well as the effects of syn- and posteruptive rock alteration, shows that basaltic terrains in Idaho and Hawai‘i provide a way to characterize the geology and major geologic substrates that host biological activity of relevance to Mars exploration. This work provides the foundation to better understand the scientific questions related to the habitability of basaltic terrains, the rationale behind selecting analog field targets, and their applicability as analogs to Mars. -
![Wednesday, March 22, 2017 [W453] MARTIAN METEORITE MADNESS: MIXING on a VARIETY of SCALES 1:30 P.M](https://docslib.b-cdn.net/cover/9633/wednesday-march-22-2017-w453-martian-meteorite-madness-mixing-on-a-variety-of-scales-1-30-p-m-489633.webp)
Wednesday, March 22, 2017 [W453] MARTIAN METEORITE MADNESS: MIXING on a VARIETY of SCALES 1:30 P.M
Lunar and Planetary Science XLVIII (2017) sess453.pdf Wednesday, March 22, 2017 [W453] MARTIAN METEORITE MADNESS: MIXING ON A VARIETY OF SCALES 1:30 p.m. Waterway Ballroom 5 Chairs: Arya Udry Geoffrey Howarth 1:30 p.m. Nielsen S. G. * Magna T. Mezger K. The Vanadium Isotopic Composition of Mars and Evidence for Solar System Heterogeneity During Planetary Accretion [#1225] Vanadium isotope composition of Mars distinct from Earth and chondrites. 1:45 p.m. Tait K. T. * Day J. M. D. Highly Siderophile Element and Os-Sr Isotope Systematics of Shergotittes [#3025] The shergottite meteorites represent geochemically diverse, broadly basaltic, and magmatically-derived rocks from Mars. New samples were processed and analyzed. 2:00 p.m. Armytage R. M. G. * Debaille V. Brandon A. D. Agee C. B. The Neodymium and Hafnium Isotopic Composition of NWA 7034, and Constraints on the Enriched End-Member for Shergottites [#1065] Couple Sm-Nd and Lu-Hf isotopic systematics in NWA 7034 suggest that such a crust is not the enriched end-member for shergottites. 2:15 p.m. Howarth G. H. * Udry A. Nickel in Olivine and Constraining Mantle Reservoirs for Shergottite Meteorites [#1375] Ni enrichment in olivine from enriched versus depleted shergottites provide evidence for constraining mantle reservoirs on Mars. 2:30 p.m. Jean M. M. * Taylor L. A. Exploring Martian Mantle Heterogeneity: Multiple SNC Reservoirs Revealed [#1666] The objective of the present study is to assess how many mixing components can be recognized, and address ongoing debates within the martian isotope community. 2:45 p.m. Udry A. * Day J.